Medical technology is a subset of medical devices, also known as medical equipment. At the same time, medical technology forms an organizational unit in hospitals.
Content
This page provides manufacturers and operators of medical technology and medical equipment with a quick overview of:
- Medical technology / medical equipment themselves
- Regulatory requirements for medical technology
- Support in complying with these requirements
1. Overview of medical technology / medical equipment
a) Terms and taxonomy
The term medical device is the umbrella term for many product categories. For example, a distinction is made between:
- Active and non-active medical devices: Active medical devices have a power supply, usually in the form of a mains connection or a battery or rechargeable battery.
- Medical devices with and without a measuring function: Manufacturers must also involve a notified body in the conformity assessment of devices with a measuring function.
Medical equipment is usually considered “physical” Programmable Electrical Medical Systems (PEMS), i.e., active medical devices. Standalone software is not classified as medical equipment.
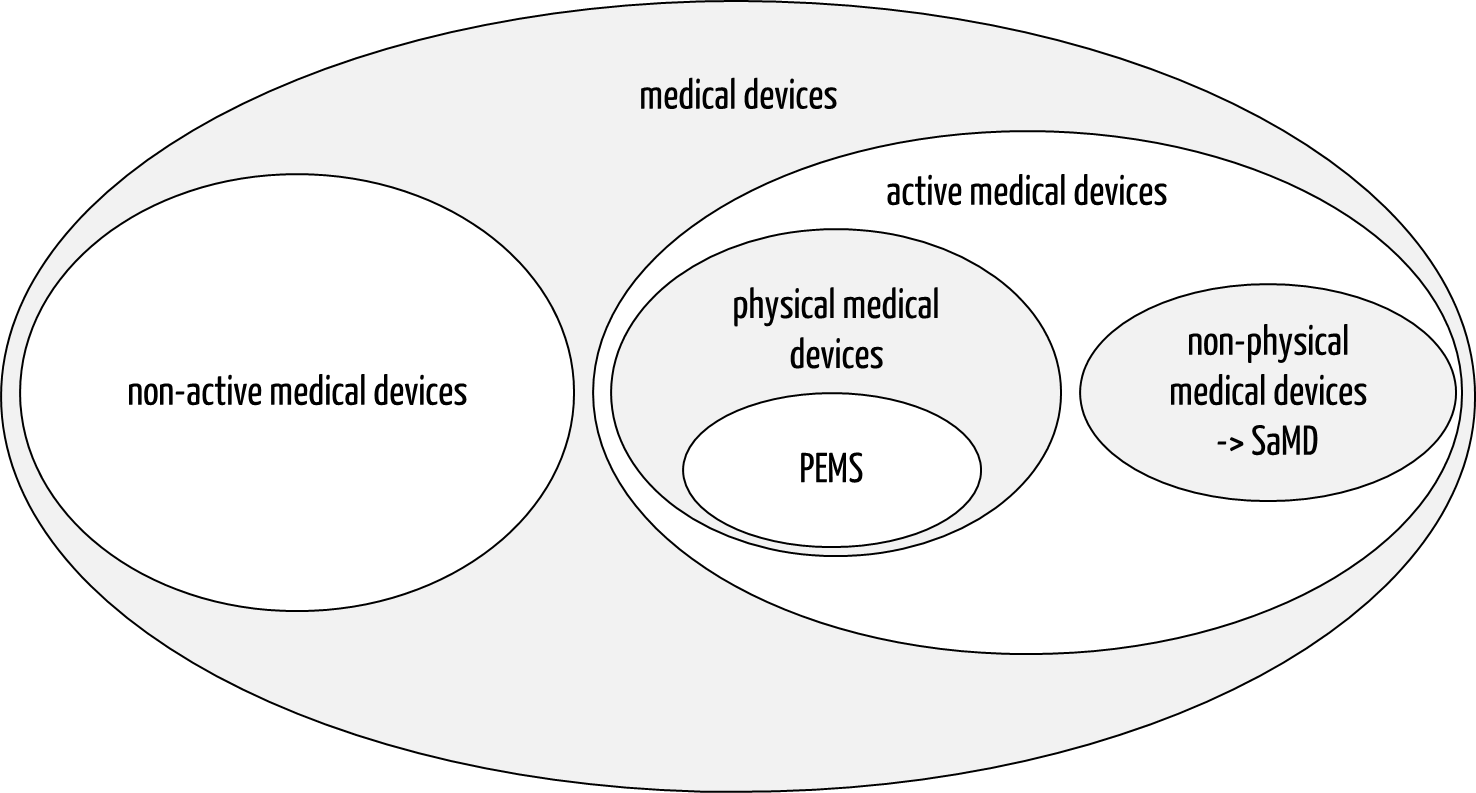
Fig. 1: In the taxonomy of medical devices, medical equipment corresponds to physical active medical devices, particularly Programmable Electrical Medical Systems (PEMS).
The term medical technology is sometimes used synonymously with the term medical equipment. Occasionally, it refers to the department in the hospital that is responsible for these medical devices.
There are often problems with the classification of clinical information systems. These are often medical devices but do not count as classic medical technology.
b) Importance of medical technology
Medical technology is an essential economic factor. BVMed, for example, provides the latest figures.
An article highlights the trends in medical technology. These include the development and use of autonomous systems and closed-loop systems.
2. Regulatory requirements for medical equipment
a) Requirements for manufacturers
As medical equipment is a subset of medical devices, all regulatory requirements for medical devices also apply to medical equipment.
Further information
The regulatory affairs page lists all laws, standards, regulations, and guidelines. In Europe, particular attention must be paid to the MDR and the MPDG.
In addition to the General Safety and Performance Requirements (GSPR), manufacturers should fulfill the requirements for medical electrical equipment and the IEC 60601 family. In addition to the requirements for electrical safety, these also include requirements for interoperability, essential performance, and functional safety.
b) Requirements for operators
Operators of medical devices and medical technology are also subject to legal requirements.
The keyword article on hospitals, laboratories, and other operators contains an overview. It refers to the requirements for installing, maintaining, and decommissioning medical devices. It also addresses specific product types, e.g., combinations of medical devices, devices combined by multiple socket-outlets, and medical device PCs.
Support
Do you still have questions about the development or operation of medical devices? Our free micro-consulting service will provide you with qualified answers.
Contact us now if you want support with developing and approving your medical devices quickly and safely.
The IEC 61010 series of standards specifies safety requirements for electrical equipment used for measurement, control, regulation, and laboratory use. The general standard IEC 61010-1 and the particular standards of the IEC 61010-2 series describe the state of the art and thus serve IVD manufacturers as a means of demonstrating compliance with the general safety…
Details
The terms maintenance, preventive maintenance, restoration, inspection, service, and safety inspections are not synonymous. But they all refer to activities in the life cycle of medical devices that serve the objective of ensuring the safety, performance, and effectiveness of these devices even after they have been placed on the market. Manufacturers and operators must meet…
Details
Directive 2014/53/EU (Radio Equipment Directive (RED)) applies to devices that use Wi-Fi or RFID, for example. Medical devices that send a radio signal because they connect to the internet or are operated remotely also need to demonstrate conformity with the RED before being marketed. In this article, learn more about
Details
Phantoms in medical technology help to develop, validate, “approve” and monitor medical devices in the markets faster and more effectively. This article describes which organizations particularly benefit from the use of these phantoms and what requirements they must meet.
Details
The term “medical device PC” is not clearly defined. However, most people understand a medical device PC to be Depending on the constellation, manufacturers must fulfill different regulatory requirements. These are presented in this article.
Details
In May 2016, the German version of IEC 60601-1-2:2014 (Edition 4) was published as DIN EN 60601-1-2:2016 with the title “Electromagnetic disturbances – Requirements and tests.” At the end of 2020, a new version of this “EMC standard” – modified by Amendment 1 and called Edition 4.1 – was published.
Details
The Machinery Directive (2006/42/EC) is generally applicable to machines of all types. However, the directive can also be relevant for medical device manufacturers: both the MDR and the IVDR refer to it. If the Machinery Directive comes into play, requirements that go beyond those of the MDR and IVDR apply. Therefore, this article will give you an…
Details
IEC 60601-1 describes essential performance as performance necessary to achieve freedom from unacceptable risk. This article aims to explain what the standard means by that and how this essential performance differs from basic safety. The article also addresses the IEC 60601-1/AMD1/ISH1:2021 INTERPRETATION SHEET 1.
Details
IEC 60601-1 defines a PESS, a Programmable Electronic Subsystem, as a system based on one or more central processing units, including their software and interfaces. The standard does not reveal what it means by system; in this context, it is a medical device component. For this, IEC 60601-1 sets out specific requirements for the PESS.
Details
