The Machinery Directive (2006/42/EC) is generally applicable to machines of all types. However, the directive can also be relevant for medical device manufacturers: both the MDR and the IVDR refer to it.
If the Machinery Directive comes into play, requirements that go beyond those of the MDR and IVDR apply. Therefore, this article will give you an overview of:
- When medical devices fall under the Machinery Directive
- Which requirements you have to take into account
- How you demonstrate conformity
- What the EU is planning for the future of the Machinery Directive
1. When you have to comply with the Machinery Directive
The Machinery Directive, published by the EU in 2006, is intended to ensure a uniform level of safety for machinery in Europe. As a general regulation for machines of all types, it deals with a wide range of hazards.
Medical devices can also be responsible for such hazards. To ensure that the uniform level of safety also applies in the case of medical devices, both the MDR and the IVDR refer to the directive.
The wording of Art. 1(12) of the MDR and Art. 1(6) of the IVDR is identical and indicates when manufacturers have to comply with the Machinery Directive:
“Devices that are also machinery within the meaning of point (a) of the second paragraph of Article 2 of Directive 2006/42/EC of the European Parliament and of the Council (35) shall, where a hazard relevant under that Directive exists, also meet the essential health and safety requirements set out in Annex I to that Directive to the extent to which those requirements are more specific than the general safety and performance requirements set out in Chapter II of Annex I to this Regulation.”
Source: Art 1(12) MDR and Art. 1(6) IVDR
The general health and safety requirements of the Machinery Directive, therefore, also apply to medical devices if the following conditions are met:
- The device is machinery according to Art. 2(2)(a) of the Machinery Directive, and
- a relevant hazard under the machinery directive may occur, and
- the Machinery Directive contains more specific requirements than the MDR or IVDR.
a) Condition 1: The medical device is considered machinery
The Machinery Directive defines “machinery” in Art. 2(2)(a).
A machine generally consists of
- an assembly
This consists of at least two parts that are connected for a specific purpose. - a drive system
At least one of the parts is driven. The driving force does not come from humans or animals but from other energy sources, such as electrical, pneumatic, hydraulic, mechanical, or thermal energy sources.
Products for lifting operations are an exception to this rule. Human power can also be the driving force in this case.
Art. 2(2)(a) defines machinery in detail as:
– an assembly, fitted with or intended to be fitted with a drive system other than directly applied human or animal effort, consisting of linked parts or components, at least one of which moves, and which are joined together for a specific application,
– an assembly referred to in the first indent, missing only the components to connect it on site or to sources of energy and motion,
– an assembly referred to in the first and second indents, ready to be installed and able to function as it stands only if mounted on a means of transport, or installed in a building or a structure,
– assemblies of machinery referred to in the first, second and third indents or partly completed machinery referred to in point (g) which, in order to achieve the same end, are arranged and controlled so that they function as an integral whole,
– an assembly of linked parts or components, at least one of which moves and which are joined together, intended for lifting loads and whose only power source is directly applied human effort;
Examples of machinery according to the Machinery Directive (that are not medical devices)
- Agricultural machinery
- Industrial robots
- Robot lawnmower
- Household appliances such as blenders or hair dryers
- Machine tools
Examples of medical devices that are also machinery according to the Machinery Directive
- The moving part of a tumor irradiation unit (gantry)
- Surgical instruments such as drills or saws
- Dental surgical instruments
- Movable operating tables
- Surgical robots
- Centrifuges
- Electrically adjustable patient beds
- Heart-lung machine
Examples of IVD devices that are also machinery according to the Machinery Directive
- Autosampler
- Centrifuges
- Sorting machines
- Feeders
b) Condition 2: A relevant hazard can occur
If the medical device falls under the definition “machinery,” the MDR and IVDR establish another condition that must be met for the Machinery Directive to apply, namely a relevant hazard must exist as a result of using the device.
A hazard is
“a potential source of injury or damage to health”
Source: Annex I, 1.1.1(a) Machinery Directive
The specific types of hazards covered by the Machinery Directive are listed in Annex I. If the medical device generates a hazard listed there, the second condition is also met.
c) Condition 3: The Machinery Directive contains more specific requirements
As a final requirement for the Machinery Directive to apply to medical devices, the regulations in Annex I of the Machinery Directive must be more specific than those in Annex I Chapter II of the MDR or IVDR.
Which requirements are more specific has to be decided on a case-by-case basis by comparing the essential requirements of the Machinery Directive with those of the MDR and/or IVDR.
Requirements that are regulated more precisely in the MDR and IVDR than in the Machinery Directive
MDR
- Biological and chemical hazards
- Hazards associated with software
- Hazards associated with usability
- Hazards due to radiation
- Electrical hazards
IVDR
- Robotic systems used to automate an IVD kit (liquid handling robotics)
- Microscopes with movable slide tables and automatic lenses
In these cases, manufacturers must comply with the MDR/IVDR.
Requirements that are regulated in more detail in the Machinery Directive than in the MDR or IVDR
- Mechanical hazards
- Safety and reliability of control systems and protective devices
- Risks from moving parts
- Information and warnings
In these cases, manufacturers must comply with the Machinery Directive.
2. What requirements does the Machinery Directive place on medical devices?
a) Only the specific requirements in Annex I are relevant
If medical devices fall under the Machinery Directive, the specific requirements of the directive apply to them.
However, manufacturers are not required to check the entire directive. Both the MDR and the IVDR only refer to Annex I of the Machinery Directive. This means:
- They only have to take Annex I of the Machinery Directive into account.
- They only have to take into account the requirements that go beyond those of the MDR or IVDR (see condition 3).
b) Risk assessment
In Annex I, the Machinery Directive lists the specific requirements for products. As a first requirement, the directive states that the relevant risks must be identified and assessed.
For the risk assessment, the Machinery Directive establishes specific requirements. However, they are in line with those of the MDR or IVDR.
They oblige the manufacturer to:
– determine the limits of the machinery, which include the intended use and any reasonably foreseeable misuse thereof,
– identify the hazards that can be generated by the machinery and the associated hazardous situations,
– estimate the risks, taking into account the severity of the possible injury or damage to health and the probability of its occurrence,
– evaluate the risks, with a view to determining whether risk reduction is required, in accordance with the objective of this Directive,
– eliminate the hazards or reduce the risks associated with these hazards by application of protective measures, in the order of priority established in section 1.1.2(b).
Source: Machinery Directive Annex I, General Principles
c) Health and safety requirements
The Machinery Directive distinguishes between health and safety requirements that apply to all machinery and those that only apply to specific machinery.
General health and safety requirements
The first part of Annex I of the Machinery Directive contains the general requirements that manufacturers must always take into account.
These are essentially the principles of safety integration that also apply to medical devices:
- Eliminate or reduce risks
- Take the necessary protective measures in relation to risks that cannot be eliminated
- Inform users of the residual risks
The general part also specifies further requirements for:
- Usability
- Accompanying documents and information
- Warnings and labeling
- Operating instructions
These requirements are also already familiar to medical device manufacturers from other regulations.
Specific health and safety requirements
The other parts of the Annex refer to specific hazards. They, therefore, only apply to machines for which the hazards listed in them can occur.
Specific hazards include:
- Hazards due to the mobility of machinery
- Hazards due to lifting operations
- Machinery intended for underground work
- Hazards due to the lifting of persons
Therefore, manufacturers must take both the first part as well as the other parts of the Annex into account. They should check which hazards apply to their product when doing so. This will then tell them which requirements they must meet according to the Machinery Directive.
3. How to demonstrate the conformity of your device
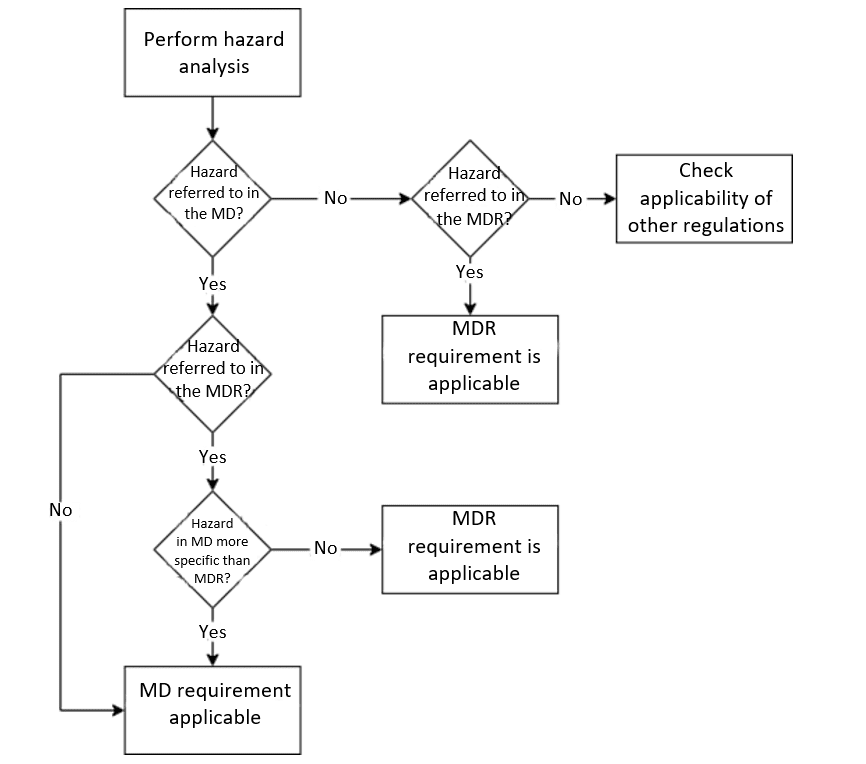
If your medical device might be machinery as defined by the Machinery Directive, follow these five steps to demonstrate conformity:
a) Step 1: Check whether your device is classed as machinery
First, check whether your device falls under the definition of machinery given in Art. 2(2)(a). If not, the Machinery Directive is not applicable.
If your device falls under the definition of machinery, then:
b) Step 2: Identify hazards
Check which hazards can be caused by your device. Among other things, this determines which requirements apply.
c) Step 3: Determine the relevant requirements
Compare the requirements in the regulations:
Go through Annex I Chapter II of the MDR or IVDR and identify which of the hazards generated by your device are mentioned.
Then go through Annex I of the Machinery Directive and note which of the hazards specified there apply to your device.
Now you have to compare:
- Does your device not cause any of the hazards listed in the Machinery Directive? Then the Machinery Directive does not apply; the MDR and/or IVDR apply.
- Do both the MDR/IVDR as well as the Machinery Directive contain hazards that your device may cause? Then you have to compare the requirements. The more precise requirement is the one that applies to you.
If the Machinery Directive regulates the hazards caused by your device more precisely, you must comply with the requirements set out in the Machinery Directive (see section 2 above).
Special case: IVDD devices
According to the IVDD, manufacturers of laboratory equipment for medical purposes that is also machinery do not have to take the Machinery Directive into account.
However, the IVDR has now brought the Machinery Directive within its scope. Manufacturers of IVDD devices must check, based on the state of the art, whether the Machinery Directive contains new requirements that are not covered by previous IVDD-specific standards.
This means that manufacturers of IVDD devices should act quickly as changes can still be made under the IVDD until the IVDR enters into force on May 26, 2022. With a valid IVDD certificate, you can benefit from the transitional periods.
If you are unsure, speak to your notified body or the Johner Institute.
d) Step 4: Identify relevant standards
Some requirements in Annex I of the Machinery Directive can be partially implemented and checked without reference to standards. There are more than 800 sector-specific standards for the remaining requirements that manufacturers can refer to when demonstrating conformity.
The current harmonized standards with presumption of conformity are published in the Official Journal of the European Union (as of Official Journal 2017) or can be found via the Regulatory Radar.
Read more about harmonized standards in the article on harmonized standards.
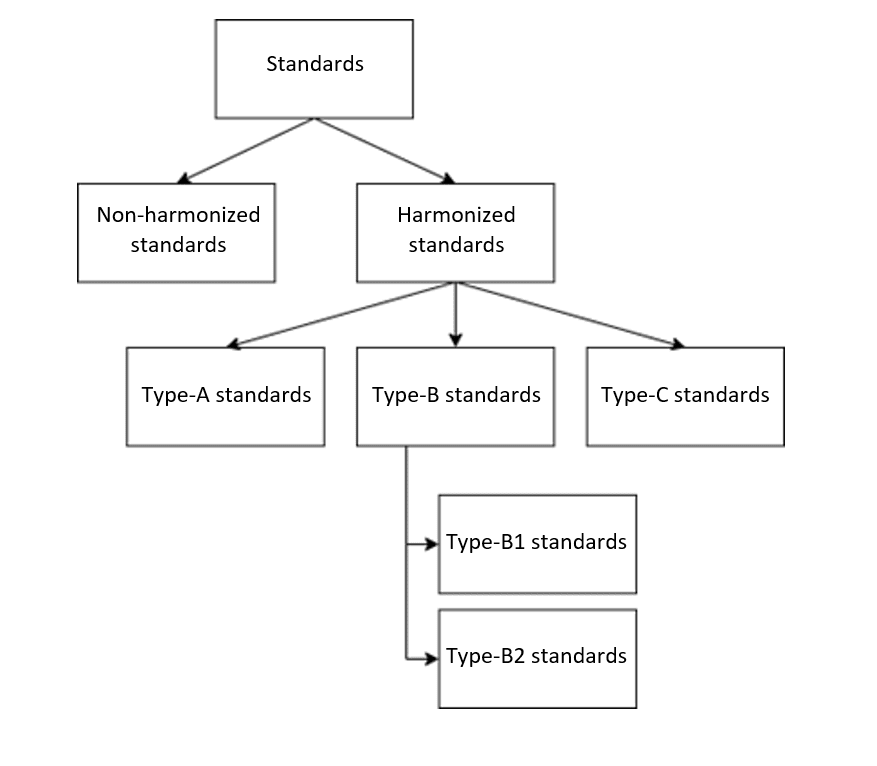
Standards framework
The Machinery Directive distinguishes between the following types of standards:
- Type-A standards: Basic safety standards with general design principles and definitions (e.g., EN ISO 12100 “Safety of machinery – General principles for design – Risk assessment and risk reduction”)
- Type-B standards: Generic safety standards that deal with one safety aspect or one type of safeguard for machinery
- B1 standards: Safety aspects (e.g., EN ISO 13854:2019 “Safety of machinery – Minimum gaps to avoid crushing of parts of the human body”)
- B2 standards: Safeguards (e.g., EN ISO 13851:2019 “Safety of machinery – Two-hand control devices – Principles for design and selection”)
- Type-C standards: Machine safety standards for a particular machine or group of machines (e.g., EN ISO 4254-11:2010 “Agricultural machinery – Safety – Part 11: Pick-up balers”)
There are no specific type-C standards for medical devices. Therefore, only the type-B standards, if applicable, are relevant for manufacturers of medical devices.
Non-harmonized standards
For some medical applications, e.g., wheelchairs and medical stairlifts, there are device-specific safety standards that are not harmonized under the MDR, IVDR, or Machinery Directive. Nevertheless, these standards can be used to assess conformity.
However, in contrast to the harmonized standards, there is no automatic presumption of conformity with regard to the medical device’s conformity with the general requirements of the MDR. That means manufacturers must show which sections of the standard are suitable as evidence of compliance with a specific requirement of the MDR or Machinery Directive. (Harmonized standards come with a ready-made comparison in Annex ZZ).
IVD devices
The following standards, among others, are relevant for IVD medical devices:
- IEC 61010-1 “Safety requirements for electrical equipment for measurement, control, and laboratory use Part 1: General requirements”
- IEC 61010-2-101 “Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment”
Section 7 of IEC 61010-2-101 covers a lot of mechanical hazards.
e) Step 5: Perform risk management
Manufacturers must perform the benefit-risk analysis, as described in Annex I of the MDR and IVDR.
The harmonized standard for performing this benefit-risk analysis is ISO 14971. This defines a risk management process that must be followed. In the vast majority of cases, the ISO 14971 process also meets the requirements of the Machinery Directive. Therefore, this is the first choice for medical device manufacturers.
In the rare cases where the ISO 14971 process does not meet the requirements of the Machinery Directive, manufacturers can switch to EN ISO 12100 instead. Its process model consists of risk analysis, risk assessment, and risk reduction and is the same as in ISO 14971.
4. The directive becomes a regulation
The EU Commission published the new Machinery Regulation on June 26, 2023, which is intended to replace the Machinery Directive.
a) Why is a Machinery Regulation needed?
The current version of the Machinery Directive was published in 2006. According to the explanatory memorandum of the draft regulation, important new technologies, with new risks, have emerged since then.
This is referring, above all, to Artificial Intelligence (AI), the regulation of which is currently a major concern for the EU. The Commission has also published a draft AI regulation in 2021. Read more about the AI Regulation in this article.
The regulation is also intended to eliminate legal uncertainties due to a lack of clarity on the scope of the existing Machinery Directive and its inconsistent definitions.
b) What this means for manufacturers
Unlike a directive, which is only addressed to the EU member states, a regulation applies directly to manufacturers.
The EU is also planning changes in the new regulation to address the problems mentioned. Therefore, manufacturers should prepare themselves for some innovations, for example, with regard to machinery and software and demonstrating conformity.
c) Timeline
For manufacturers, the question arises as to whether the Machinery Directive or the Machinery Regulation applies. Article 51(2) of the Machinery Regulation (EU) 2023/1230 states that Directive 2006/42/EC will be repealed as of January 14, 2027. In addition, the transitional periods according to Article 52 of the Regulation apply.
5. Conclusion
With the detour via the Machinery Directive, the MDR and IVDR impose additional requirements on some medical devices that are also considered machinery. Initially, this does mean additional work checking the requirements.
When it comes to demonstrating conformity, however, manufacturers can, for the most part, use the familiar standards in the context of the Machinery Directive as well.
The Johner Institute can help you with any questions you might have on conformity requirements for medical devices that fall under the Machinery Directive. Simply contact us by email or via the contact form.


