Defining the regulatory strategy is one of the central tasks of the regulatory affairs departments at medical device manufacturers.
Why is a good regulatory strategy so important? What do regulatory affairs experts need to do? This article provides the answers.
The Johner Institute’s free Regulatory Strategy Kit will help you,
- identify regulatory differences in the various markets,
- check the completeness of your regulatory strategy and thus avoid mistakes, and
- document your regulatory strategy compactly and understandably for management.
1. What does a regulatory strategy determine?
a) Background
Manufacturers of medical devices must comply with a large number of regulatory requirements in order to legally market their medical devices worldwide. These include
- legal requirements,
- normative requirements, and
- authority guidelines.
These differ worldwide, as do the approval procedures.
b) Objectives
The regulatory strategy should help to ensure that devices can be brought to market quickly, safely, and without unnecessary effort.
A more complete overview of the objectives of a regulatory strategy can be found below in the chapter “Why is the regulatory strategy so important?”.
To achieve this, manufacturers must do the following:
- identify regulatory requirements and other factors that influence approval
- make regulatory-relevant decisions
The next subchapter explains how these activities influence each other (see Fig. 1).
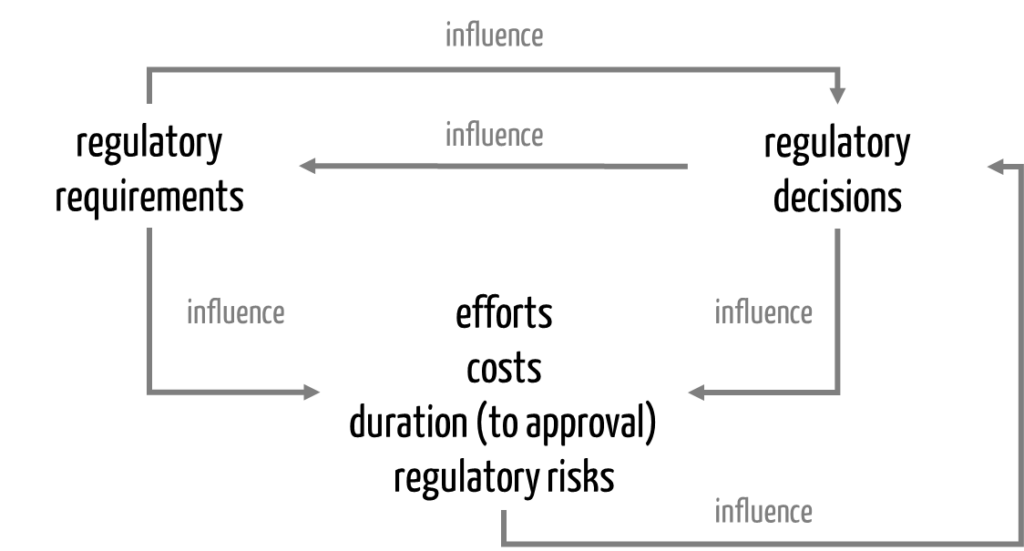
c) Interaction of regulatory requirements and decisions
During the development of a regulatory strategy, manufacturers gain certainty about the regulatory requirements for their device. These requirements, in turn, depend on the regulatory decisions they make (see Tab. 1).
| regulatory requirements and other factors | influence through regulatory decisions (examples) |
| qualification of the medical device | destination country intended use definition of what qualifies as a device choice of medical device vs. accessory |
| risk classification | (restriction) of the intended use (e.g., the indication) definition of what qualifies as a device restriction of functionality choice of technology, etc. |
| path to market release (approval, registration), special procedure if necessary | destination country intended use selection of the (possible)approval procedure |
| necessary documents (technical documentation) | selected approval procedure |
| identification of equivalent products | destination country intended use definition of what qualifies as a device selected approval procedure |
| necessary (clinical) data or clinical investigation | intended use functionality, technology selected approval procedure selected equivalent device |
| product-specific requirements (laws, standards, guidelines, etc.) | destination country intended use functionality, technology selected approval procedure |
| instructions for use, labeling | destination country intended use |
| QM systems including post-market surveillance | destination country |
d) Further regulatory decisions
Manufacturers make some decisions to influence what regulatory requirements apply to them and their devices. Other decisions have a direct impact on the effort, costs, approval times, and regulatory risks:
- sequence of markets into which the devices are to be placed on the market
- combined approval of several devices in one approval procedure (“bundling”)
- choice of (local) partners (e.g., for registration and authorization)
- interaction with the authority (e.g., through the benefit of the Q-Submission program)
With the checklist from the Johner Institute’s Regulatory Strategy Kit, manufacturers of medical devices can check the completeness of their regulatory strategy. The kit also contains a mind map of the most important differences in regulatory requirements in the various markets.
The following two images show excerpts.

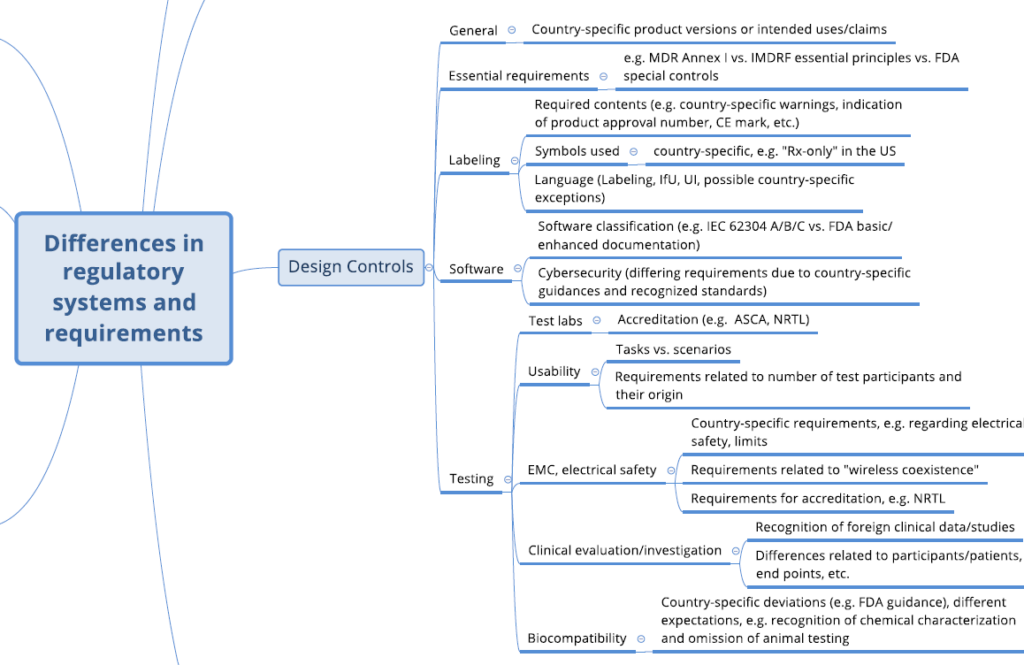
2. Why is the regulatory strategy so important?
A suitable regulatory strategy is indispensable for companies for six reasons:
| significance, benefit | explanation, examples |
| 1. Plannability of costs and durations | Planning is necessary in order to provide the necessary resources and estimate costs. It is therefore an important input for business and marketing plans. |
| 2. Regulatory clarity | If the company management has clarity about the regulatory requirements, it can make strategic decisions, e.g., about international marketing. |
| 3. Minimization of time and costs | By adapting the intended use, bundling products into one single submission and taking dependencies into account, internal and external efforts (e.g., for collecting clinical data) and costs can be minimized, and approval procedures accelerated. |
| 4. Fast revenues | The earlier a device is launched on the market, the faster manufacturers can generate revenue. Revenues are usually high for new devices as long as the competition has not yet caught up. |
| 5. Minimizing regulatory risks | A flawed strategy can have serious consequences. If the manufacturer is only informed by the authority during the approval process that the chosen approval route is not working, they loses time and money and the competition overtakes it. |
| 6. Plannability of development | Without knowledge of country-specific requirements at the start of development, there is a risk that changes will have to be made to the device design after “design freeze” or that additional testing will have to be conducted. |
The conclusion is that a regulatory strategy is essential for the company’s competitiveness. The responsibility of regulatory affairs experts is correspondingly high.
3. How do you develop a regulatory strategy?
a) Ensure expertise
Defining a regulatory strategy is no trivial task. The process requires a great deal of expertise, both in product-specific terms (e.g., with regard to the medical purpose or the technologies used) and in regulatory and clinical terms.
Depending on the target country, this also implies linguistic expertise, as in many countries, the legal requirements are only available in the local language.
Ideally, you will form a team or have access to product management, clinical experts, regulatory affairs, and quality management.
If this expertise does not exist or is not sufficient to define a robust regulatory strategy, then get external support such as that of the Johner Institute.
b) Start early
Start developing the strategy in good time, preferably very early on in the product development process. If you know the regulatory requirements of the target countries right from the start, you can develop the product in such a way that the country-specific requirements are met straight away, and product tests are combined across countries. This saves you additional testing efforts or studies later on.
c) Proceed in the right order
Collect input
The following serve as input for the strategy:
- product description, including intended use
- list of target countries
- technical documentation, if available
- information on existing approvals
Quality and classify your product
Start at the top level and determine the qualification, classification, and possible approval procedures for each target country.
Adapt intended use if necessary
Discuss the intended use and claims. If there are options to choose a less complex approval procedure, but the intended use has to be adapted in return, you should discuss this with your clinical experts.
In general, the intended use has an influence on the necessary clinical evidence. Therefore, never consider the clinical and regulatory requirements separately but rather develop a combined approval strategy. Only then can you be sure that you will not encounter unexpected regulatory hurdles.
Identify country-specific requirements
In the next step, identify the country-specific regulatory requirements. Compare these and identify the differences and similarities (e.g., with regard to the basic requirements).
Create a summary for the management
Summarize your results and the most important aspects of your strategy for management, e.g., as a presentation or management summary. A regulatory strategy can be very extensive!
d) Review strategy on a regular basis
Review your regulatory strategy at regular intervals. Adjust it if there are changes, be they technological, regulatory, or those resulting from an overarching business plan.
4. Summary and conclusion
The regulatory strategy is an indispensable basis for market access and, thus, for entrepreneurial success. In the worst case, an inadequate strategy can mean the end of your business if, for example, you lose time and money due to an incorrect approval route and the competition is faster.
You should, therefore, place great importance on the development of a regulatory strategy. Take the time to develop it and start as early as possible in the product development process.
If you do not have the necessary expertise or would like a second opinion, our regulatory and clinical experts will be happy to help you develop or review your regulatory strategy.
The Johner Institute’s free Regulatory Strategy Kit will help you,
- identify regulatory differences in the various markets,
- check the completeness of your regulatory strategy and thus avoid mistakes, and
- document your regulatory strategy compactly and understandably for management.


