Poor instructions for use are a common cause of use errors that can result in harm to patients and users. Furthermore, instructions for use for medical devices and IVDs are subject to strict regulatory requirements. The MDR, the IVDR, the FDA and numerous standards establish specific requirements for the instructions for use.
This article will explain how to write instructions for use that:
- help prevent harm to patients, users, or third parties,
- fulfill all relevant regulations and, therefore, pass the audit,
- are user-friendly and, therefore, increase customers satisfaction and your market success.
1. Definition and synonyms
“means the information provided by the manufacturer to inform the user of a device’s intended purpose and proper use and of any precautions to be taken;”
“package insert; portion of the accompanying information that is essential for the safe and effective use of a medical device or accessory directed to the user of the medical device”
“General and technical information provided by the manufacturer to inform the user of the medical device or IVD medical device’s intended purpose and proper use and of any contraindications, warnings, or precautions to be taken. It is provided by the manufacturer to support and assist the device users in its safe and appropriate use.”
As you can see, instructions for use are generally printed documents accompanying the device intended to inform the user of the intended purpose, safety information, and correct use.
The following terms are often used synonymously:
- Directions for use
- Manual or user manual
- Operating manual
- User guide
All stand for the same.
2. Regulatory requirements for instructions for use
Numerous laws, ordinances, directives, standards, and other regulations establish requirements for instructions for use. These include:
| Regulation | Description |
| Medical Device Regulation (MDR) | Most of the requirements for the instructions for use can be found in Annex I, Section 23. |
| In Vitro Diagnostic Medical Device Regulation (IVDR) | Most of the requirements for the instructions for use can be found in Annex I, Section 20. |
| Regulation 2021/2226 on electronic instructions for use | Regulation 2021/2226 governs the conditions under which instructions for use may be made available electronically (eIFU) and the associated requirements. The regulation is currently being revised to allow eIFUs also for lay users of software devices (see here). |
| IEC 62366-1 | The instructions for use are part of the user interface. Therefore, they must be developed following a usability engineering process according to IEC 62366-1. |
| IEC 60601-1 | Section 7.9 specifies what has to be included in instructions for use for medical electrical equipment. |
| ISO 20417 | ISO 20417 replaces EN 1041 and, in Section 6.6 in particular, establishes requirements for instructions for use. |
| IEC 81001-5-1 | Annex E specifies what content must be communicated to the user with regard to cybersecurity. |
| ISO 18113-(1-5): In vitro diagnostic medical devices — Information supplied by the manufacturer – Part 1: Terms, definitions and general requirements – Part 2: In vitro diagnostic reagents for professional use – Part 3: In vitro diagnostic instruments for professional use – Part 4: In vitro diagnostic reagents for self-testing – Part 5: In vitro diagnostic instruments for self-testing | Parts 1-5 of ISO 18113 specify the requirements that IVD instructions for use must meet. |
| IMDRF/GRRP WG/N52 FINAL:2019: Principles of Labelling for Medical Devices and IVD Medical Devices | The aim of this IMDRF/GRRP document is to establish internationally harmonized requirements for labeling (including instructions for use). |
| FDA: 21 CFR part 801 and 809 | The FDA has a series of requirements for the instructions for use; in 21 CFR part 801 for medical devices and in part 809 for IVDs. |
| FDA Guidance on Medical Device Patient Labeling | Medical device manufacturers should follow this guidance for device clearing in the USA. It also provides helpful tips for writing instructions for use outside the USA as well. |
| FDA Guidance: Applying Human Factors and Usability Engineering to Medical Devices | Analogous to 62366-1, manufacturers should follow this guidance when writing instructions for use for the US market. |
| MDCG 2019-16 | Chapter 4 deals with the requirements for instructions for use with regard to cybersecurity. |
| FDA Cybersecurity Guidance Documents | The FDA has dedicated two guidance documents to cybersecurity and the corresponding instructions for use. |
a) Best practice guides for creating instructions for use
Other standards and guides provide advice on how to write good instructions for use:
- IEC/IEEE 82079-1: IEC/IEEE 82079-1 (the successor to DIN EN 62079) is as comprehensive as it is practical. Even though this standard is not focused on one particular sector, it applies to medical devices as it is the state of the art for preparing instructions for use.
- AAMI TIR 49: In contrast to 82079-1, AAMI TIR 49 focuses on medical devices and provides guidance for the “Design of training and instructional materials for medical devices used in non-clinical environments.”
- ANSI Z535: ANSI Z535 specifies how safety information should be designed, for example how symbols and colors should be used.
- The WHO Guidance: Designing instructions for use for in vitro diagnostic medical devices also provides valuable tips on how to prepare instructions for use for devices that are not IVDs. It even provides a structure and contains text templates.
- The Medical Device and Health IT Joint Security Plan version 2 (JSP2), which is also referenced by the FDA, provides a good basic structure for creating cybersecurity documentation in Annex D.
See chapters 4 and 5 for more information on the content of these guidances.
b) When instructions for use are required and when they are not
MDR requirements
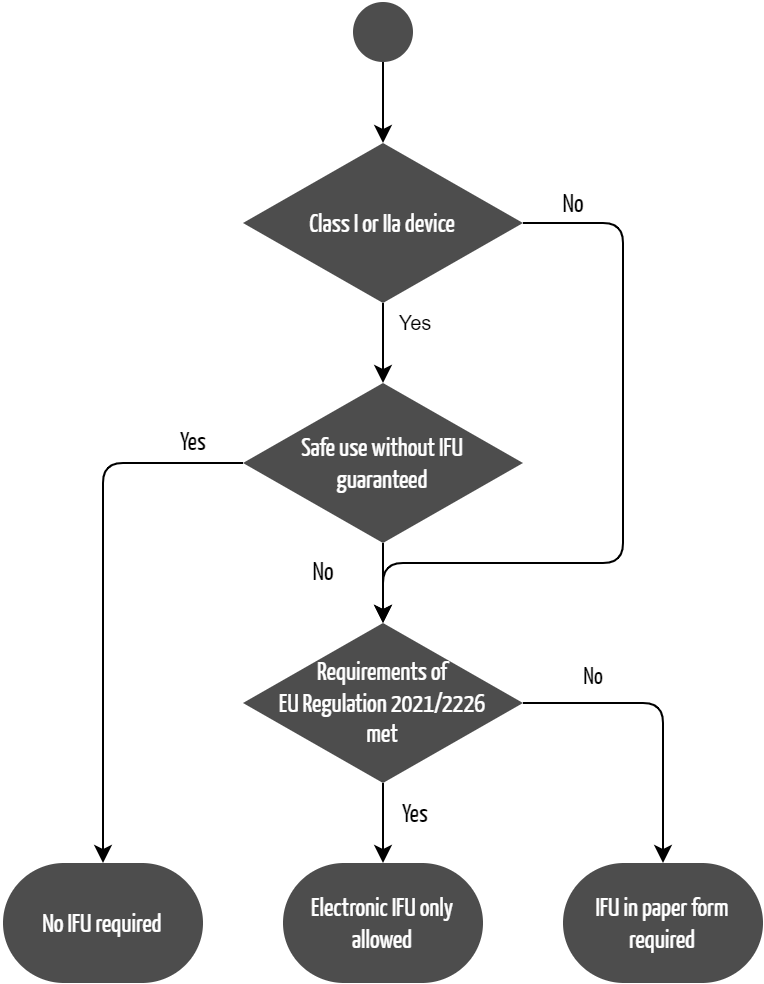
The Medical Device Regulation (MDR) permits to provide no instructions for use under certain circumstances (see Fig. 1). In Annex I, Section 23.1(d), it states:
“Instructions for use shall be provided together with devices. By way of exception, instructions for use shall not be required for class I and class IIa devices if such devices can be used safely without any such instructions and unless otherwise provided for elsewhere in this Section.”
Evidence that the device can be used safely even without the instructions for use can be provided in two ways:
- The manufacturer’s risk analysis according to ISO 14971 shows that misuse of the device does not result in a hazardous situation for users or patients. This implies that the device can be used safely even without instructions for use. However, in this case, there is a risk that potential use errors werenot taken into account in the risk analysis. Manufacturers don’t always take every possible use error into account and, in such cases, only observe these use errors in usability tests.
- This means usability tests are a better way of providing this evidence. If the intended users in the intended use environment do not make any use errors in usability tests, this is objective evidence that the device can be used safely without instructions for use.
According to the IVDR
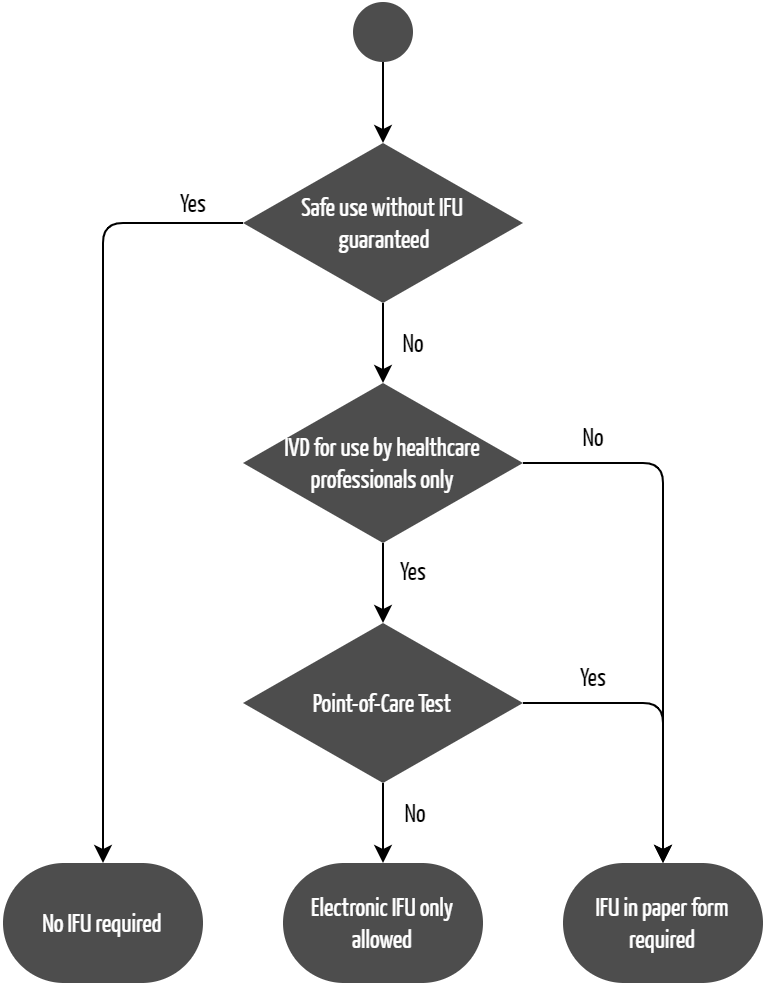
Similarly, instructions for use are also not required under certain conditions for IVDs (see Fig. 2). However, unlike the MDR, the IVDR does not make this exemption dependent of the class of the device. In Annex I, Section 20.1(d), the IVDR states:
“Instructions for use shall be provided together with devices. However, in duly justified and exceptional cases instructions for use shall not be required or may be abbreviated if the device can be used safely and as intended by the manufacturer without any such instructions for use.
c) How should the instructions for use be provided and in what quantity?
As a general rule, the instructions for use must be provided in paper form. Exceptions are possible under certain conditions (see chapter 5d).
MDR and IVDR both require the instructions for use to be provided with the device.
If the manufacturer supplies several products to a single user and/or location, a single copy of the instructions for use is sufficient if the following conditions are met:
- The provision of only one copy of the instructions for use has been agreed in advance with the buyer and
- copies can be delivered at any time on request and free of charge.
Providing only one copy of the instructions for use is permitted under the same conditions under the IVDR. The prerequisite is that the devices are not for self-testing or point-of-care testing.
Instructions for use do not have to be one (bound) document. In some cases, it may even be better to split the instructions for use into several documents, e.g., into one version specifically for non-professionals and one for professional users or service technicians.
d) Provision as electronic instructions for use (eIFU)
Under the MDR, the manufacturer has the option of providing the instructions for use in electronic form only. Regulation 2021/2226 on electronic instructions for use specifies when instructions for use can be provided in electronic form instead of in paper form.
Read more on the subject of electronic instructions for use and Regulation 2021/2226 here.
EU Regulation 2021/2226 on electronic instructions for use does not apply to IVDs. Instead, Annex I, Chapter III, Section 20.1(f) of the IVDR regulates when manufacturers do not have to provide the instructions for use in paper format:
“When the device is intended for professional use only, instructions for use may be provided to the user in non-paper format (e.g. electronic), except when the device is intended for near-patient testing.”
e) Contents of instructions for use
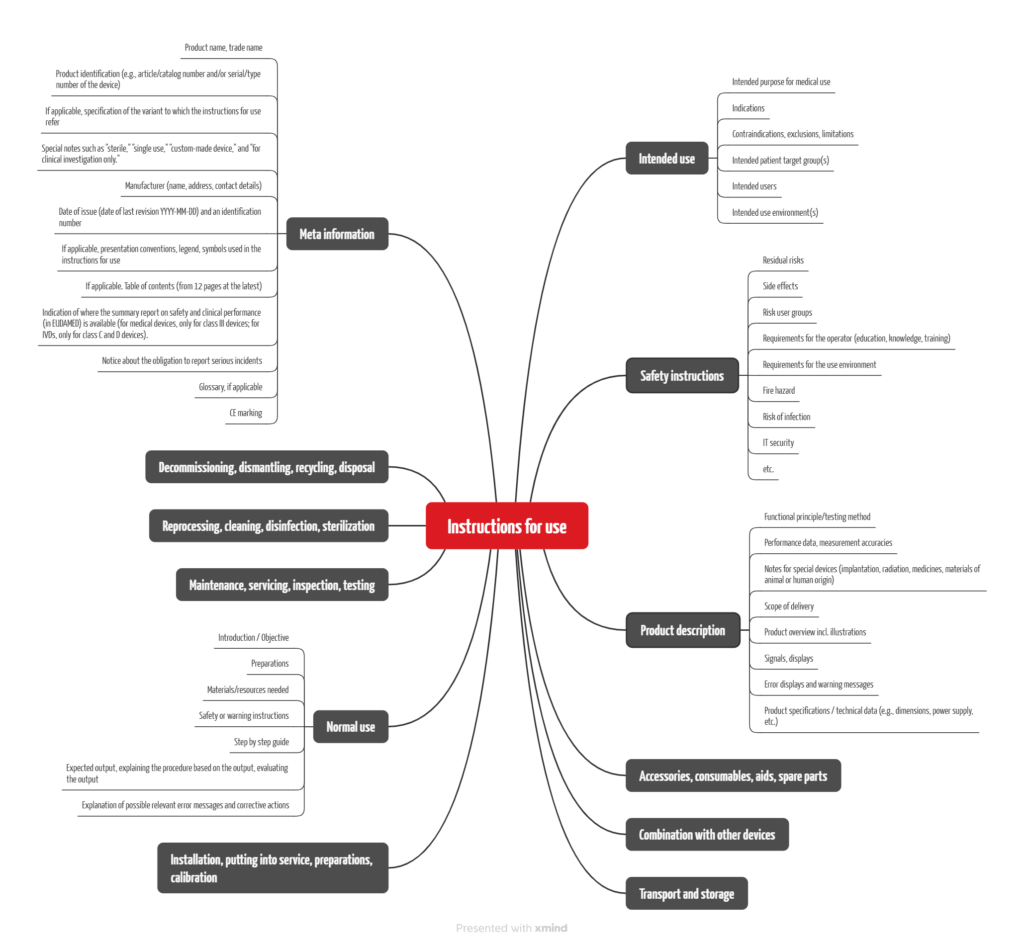
If we summarize the requirements of the regulations mentioned above, we find that the main contents required are:
f) Language requirements of instructions for use
Article 10(11) of the MDR and Article 10(10) of the IVDR require the instructions for use to be provided in one or more of the official language(s) of the target country.
Belgium, for example, requires that the instructions for use be available in German AND Dutch AND French – or in English for professional users who can request a language of their choice free of charge.
In January 2024 – 7 years after the publication of the MDR – the European Commission finally published an overview of all national language requirements in the EU. This is very helpful and, in many cases, also links directly to the relevant legislation. The graphical user interface is also explicitly mentioned. Unfortunately, the editors seem to have lost a little motivation during the writing process to search through the original laws (Hungary), translate them, and check the content for correctness (Lithuania) or complete the table (Spain).
Contact our consultants for an up-to-date list of language requirements for instructions for use and labeling for medical devices and IVDs within the EU. You can use it as a supplement to the European Commission’s list if necessary.
In Germany, the Medizinproduktedurchführungsgesetz regulates the language issue:
8(2) “Devices may only be supplied to users and patients within the scope of this law if the information intended for users and patients is provided in German. In justified cases, the information may also be provided in English or another language easily understood by the user of the medical device, provided this information is intended exclusively for professional users and the safety information is also provided in German or in the user’s language.”
In Switzerland, the instructions for use must always be provided in German AND French AND Italian, regardless of the language area. They can be provided to professionals in fewer than the three official languages or in English provided that the professional agrees and no risks result from this (see MepV Art. 16).
g) Requirements for the translation of instructions for use
Neither the MDR nor the IVDR establish any explicit requirements for the translation of the instructions for use. However, the instructions for use must be comprehensible for the intended users in all target languages. Annex I, Section 23.1(a) of the MDR states:
“In particular, instructions for use shall be written in terms readily understood by the intended user […].”
This places special demands on the translation of the instructions for use from the original language to the target language.
IEC/IEEE 82079-1 and the quality standard for translation service providers ISO 17100:2015 establish requirements for the translation process and linguists. For example, the linguist should have the following skills:
- They should have theoretical knowledge and practical experience of translating medical texts.
- They should be fluent in the original language.
- They should be a native speaker of the target language.
- They should have a certain minimum experience in the field of the text to be translated and must be familiar with the product-specific terminology.
The requirement that the linguist is a native speaker of the target language is likely to be difficult for manufacturers to meet by themselves given there are 24 official languages in the EU. So, we recommend using translation service providers.
The translation service provider should have experience in the field of medical devices, a certified quality management system according to ISO 9001:2015, ISO 17100:2015 or ISO 13485:2016, and also specialize in instructions for use within the scope of the MDR/IVDR.
3. Process of preparing the instructions for use
IEC 62366-1 and the FDA’s “Human Factors Engineering” guidance require a process for developing a user interface (UI). The instructions for use are an integral part of the user interface. Therefore, manufacturers must also apply the usability engineering process to the instructions for use. This means conducting usability testing of the instructions for use with representative users.
Find out more about the usability engineering process according to 62366-1 in our articles IEC 62366-1:2015 usability standard news (German) and Summative evaluation: What you need to pay attention to.
In addition, both IEC 82079-1 and AAMI TIR 49 provide specific recommendations for establishing a process for creating the instructions for use.
The process for creating the instructions for use should include the following steps:
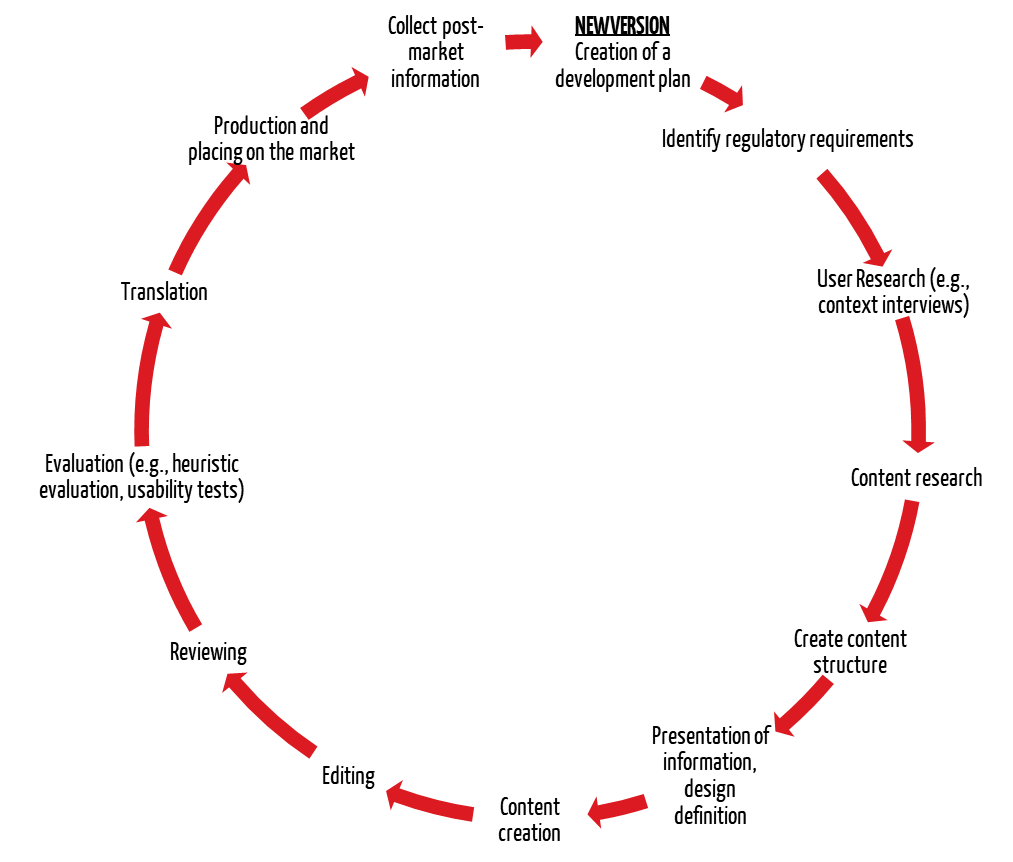
The process should be applied iteratively. In other words, an evaluation should be carried out at important milestones and the results of the evaluation should serve as input for further development.
4. Typical mistakes and their consequences
a) Mistakes that the Johner Institute’s consultants and auditors regularly encounter
- The information in the instructions for use are incorrect.
- The information doesn’t marry up with the device (anymore) – this is particularly common after software updates.
- Which device or which variants the instructions for use refer to and the version number of the instructions for use are not clear.
- Terms are not defined, consistent terminology is lacking, which encourages misunderstanding.
- The text is incomprehensible, sentences are too long, too many phrases are in the passive voice and too abstract. The text uses technical terms and is not based on the vocabulary of the target group.
- There is no structure or the structure is not clear, making navigation and orientation difficult.
- Images or other forms of visualization are missing.
- Font sizes are too small and there is no color coding or it is used inconsistently. The same applies to text sizes and text markups, as well as the page formatting and the use of elements such as symbols.
- There is no translation or the translation is poor. Google Translate is a no-go.
- There is only one single, hard-to-understand version of the instructions for use for different device variants and combinations with other devices.
- Content required for understanding and safe use is missing. In particular, the instructions for use do not cover all tasks or the complete life cycle from installation to decommissioning and disposal.
- Superfluous information, e.g., about the manufacturer, bloats the instructions for use.
- The instructions for use were not created by qualified staff but, for example, by untrained developers.
- The manufacturers do not check the effectiveness of the instructions for use, e.g., in a usability test.
- The target group is not taken into account sufficiently.
- Manufacturers do not have process for informing users on errors in the instructions for use after delivery.
b) The consequences of inadequate instructions for use
Again and again, in dozens of usability tests, our consultants have seen use errors resulting from inadequate instructions for use. Problems in usability tests caused by inadequate instructions for use can delay device authorization.
If your device has already been placed on the market with inadequate instructions for use, in the worst case they could lead to harm to patients, users, or third parties.
5. Five tips for writing instructions for use
Tip 1: Get professionals to do the job
Don’t leave the writing of instructions for use to developers or product managers who are not trained for this. You need specialized authors, editors, translators, proofreaders, illustrators, and reviewers.
Tip 2: Specify your readers and your goals
Instructions for use are not intrinsically good or bad. They must be written for the users specified in the intended purpose. Instructions for use for medical professionals will not be the same as those written for lay people or those written for service technicians.
You should write down the goals you want to achieve with the instructions for use. What use errors and therefore which risks do you want to minimize? Which tasks does safe use have to be ensured for?
Tip 3: Use a consistent structure and editorial guidelines
Consistent presentation makes reading easier. Editorial guidelines should make sure that the instructions for use contain the following:
- Subject/heading
- Description
- Aim of the action
- Prerequisite
- Status
- Action
- Result to be achieved
- Warning
- Example
- Images and tables with corresponding legends
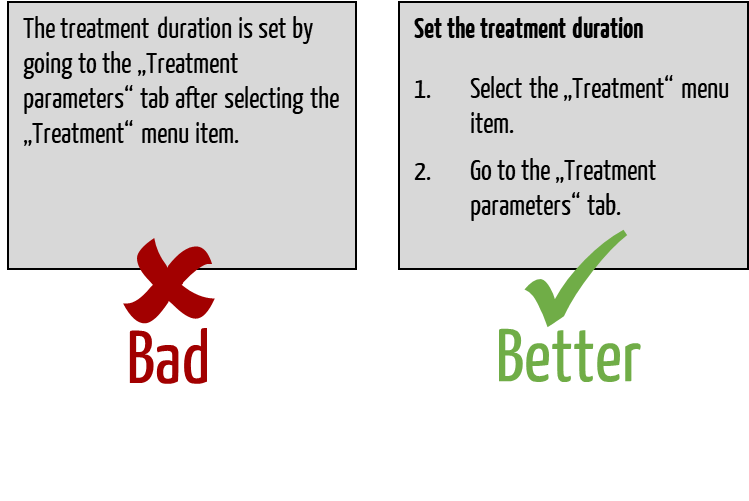
When designing instructions for use, you should at least:
- Number each individual page with Arabic numerals
- Write instructions in the imperative, in the active voice and in simple language
- Use verbs and not nouns
- Only describe one action in each step (or a maximum of three logically and closely related actions) Only give one instruction in each sentence
- If you add illustrations, they should be clearly linked to the explanatory text
Manufacturers often write instructions that don’t explain actions clearly enough.
Tip 4: Understand instructions for use as a (continuous) process
Writing instructions for use is a team sport with a lot of steps that have to be defined and planned.
Just like the device, instructions for use must be continuously improved.
Tip 5: Follow ISO 14971, IEC 62366-1 and the FDA guidance document “Human Factors Engineering”
The instructions for use are part of the device. Therefore, they are subject to the regulatory requirements governing usability. IEC 62366:2006 even has a separate section on accompanying documentation.
IEC 62366-1:2015 requires these materials to be treated in the same way as the device itself. This means their usability and the effectiveness of the actions they describe – in this case in the form of guidance in the instructions for use – also have to be evaluated.
Professional companies combine several methods:
- Usability tests / participant observations
- Questionnaires
- Self-assessments
- Expert opinions, inspections, review by experts
- Analysis of feedback on previous versions of the instructions for use
The Johner Institute often reviews instructions for use as part of formative and summative usability evaluations.
Tip 6: Cybersecurity documentation as a risk-minimizing measure
According to the Defense-In-Depth Concept, it is essential to provide users of your networking devices with sufficient information about cybersecurity in order to reduce potential risks as much as possible. The FDA, in particular, demands a high degree of transparency here, which can be achieved through detailed instructions for use. In detail, these are
- Instructions and specifications (e.g., malware protection)
- Diagrams
- A list of network ports and interfaces
- Requirements for the infrastructure
- The SBOM
- A possible download process
- The behavior of the product in the event of security incidents
- Description of the security functions
- Backup and recovery
- Description of the safety configuration after sending (change passwords, firewall settings, etc.)
- Logging mechanism
- End of Support / End of Life
- Safe disposal (for example, purging of sensitive data)
Here it explicitly refers to the Medical Device and Health IT Joint Security Plan (JSP2), which manufacturers can use as a basis for transmitting this information. Annex D can serve as a good example here, which you can adapt specifically to your product in accordance with your risk analysis – this procedure is also recommended for Europe, as the requirements largely coincide with IEC 81001-5-1.
The MDS2 form is also becoming increasingly important internationally. Many users now require it as a condition of purchase.
6. Conclusion
Writing instructions for use requires the same level of professionalism as developing the device itself. The job should not be seen as a necessary evil and quickly palmed off to people who are not sufficiently qualified for it.
The MDR, IVDR, and certain standards set requirements for the information the instructions for use must contain; specific guidance on how to write usable instructions for use can be found in other standards such as IEC 82097-1 or AAMI TIR 49.
It is essential that manufacturers remember that instructions for use have to be tested in the same way as other risk-minimizing actions, particularly those relating to usability. This means instructions for use should also be evaluated as part of the formative and summative usability evaluations as per IEC 62366-1.
The experts at the Johner Institute can help you with consulting and templates for writing and reviewing instructions for use. Please get in touch with us.
Change history
- 2024-08-23: Addition of further standards and guidance documents, adaptation of chapter 2.f), addition of a further tip in chapter 5, editorial changes
- 2022-02-18: Complete revision