More and more medical devices are using artificial intelligence e.g., to diagnose patients more precisely and to treat them more effectively.
Please refer to the overview article with the definitions of the terms artificial intelligence, machine learning, and neural networks.
Our Medical Device University offers comprehensive support through more than 25 video training courses on AI and suitable templates.
1. Applications of artificial intelligence in medicine
a) Overview
Manufacturers use artificial intelligence, especially machine learning, for tasks such as the following:
| Task | Data that can help AI with this task |
| detecting a retinopathy | images of the eye fundus |
| counting and recognizing certain cell types | images of histological sections |
| diagnosis of heart infarctions, Alzheimer’s, cancer, etc. | radiology images, e.g., CT, MRI |
| detecting depression | speech, movement patterns |
| selection and dosage of medicines | diagnoses, gene data, etc. |
| diagnosis of heart diseases, degenerative brain diseases, etc. | ECG or EEG signals |
| detecting epidemics | internet searches |
| disease prognoses | laboratory values, environmental factors etc. |
| time-of-death prognosis for intensive care patients | vital signs, laboratory values and other data in the patient’s records |
Further applications include:
- detection, analysis, and improvement of signals, e.g., weak and noisy signals
- extraction of structured data from unstructured text
- segmentation of tissues, e.g., for irradiation planning
The FDA has published an extensive list of AI-based medical devices that will be very helpful for manufacturers in order to:
- create a clinical evaluation
- search for equivalent devices
- get ideas for new devices
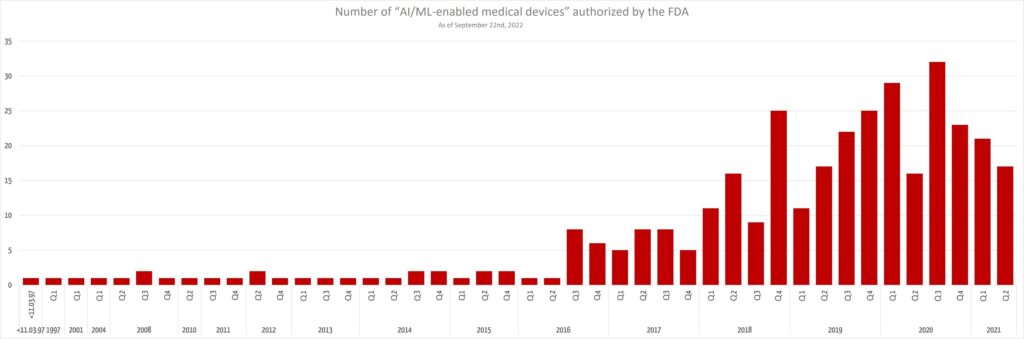
It is interesting to note that the number of newly authorized AI-based devices is not increasing any further.
b) Tasks: classification and regression
The procedures are used for the purpose of classification or regression.
Examples of classification:
- decision as to whether there is a diagnosis
- deciding whether cells are cancer cells or not
- selecting a medicine
Examples of regression:
- determining the dose of a medicine
- time-of-death prognosis
c) Algorithms / models / types
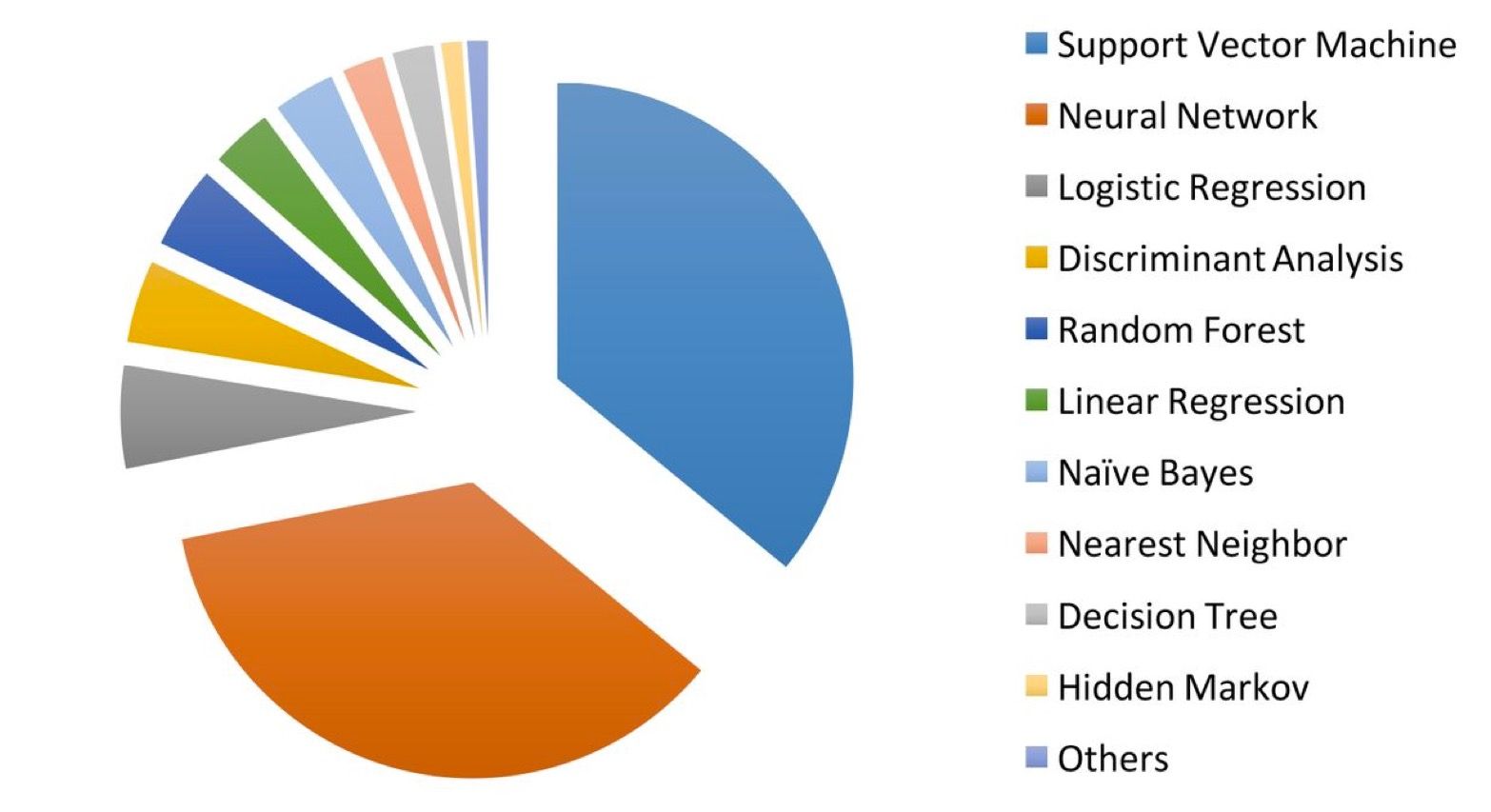
The assumption that artificial intelligence in medicine mainly uses neural networks is not correct. A study by Jiang et al. showed that support vector machines are used most frequently (see Fig. 2). Some medical devices use several methods at the same time.
The list of the most frequently and successfully used models is constantly changing. Procedures such as XGBoost, for example, have gained in popularity.
2. Conclusion, outlook
a) From hype to disillusion to actual practice
Artificial intelligence is currently receiving a lot of hype. A lot of “articles” praise it as either the solution to every medical problem or the start of a dystrophy in which machines will take over. We are facing a period of disillusionment. “Dr. Watson versagt” [“Dr. Watson fails”] was the title on article in issue 32/2018 of the German “Der Spiegel” on the use of AI in medicine.
It has to be expected that the media will write over-the-top and scandalized reports on cases where bad AI decisions have tragic consequences. But over time, the use of AI will become just as normal and indispensable as the use of electricity. We can no longer afford and no longer want to pay for medical staff to perform tasks that computers can do better and faster.
b) Regulatory uncertainty
Even if the FDA submitted a good first draft: The regulatory framework and best practices lag behind the use of AI. This leads to risks for patients (medical devices are less safe) and for manufacturers (audits and approval procedures seem to reach arbitrary conclusions).
The WHO feels compelled to focus more on the topic and develop a WHO guideline. The focus group “Artificial Intelligence for Healthcare” is evidence of this effort. Everyone is invited to participate.
This also applies to the guideline on the safe development and use of artificial intelligence in medical devices presented above. This guideline already formulates very specific requirements and thus helps manufacturers on the one hand and notified bodies and authorities on the other to achieve a uniform understanding of the state of the art and, thus, a common basis for product testing and audits.
Change history:
- 2025-01-15: All chapters except the one on the use of AI in medicine removed
- 2024-01-26: Definition of “AI system” from the AI Act added to the definitions section
- 2023-11-03: FDA regulatory requirements moved to this article
- 2023-06-27: Outlook on AI regulation given in section 3.a)
- 2021-11-07: In section 2.a) evaluation of the FDA’s list added
- 2021-10-03: In section 2.a) list of the FDA added

Great article! We always appreciate valuable insights like these. Thanks for sharing!