1. Definitions
Medical software includes all software used for healthcare, particularly for medical devices or medical devices (embedded software), and software that is itself a medical device (standalone software).
IEC/CD1 82304-1 (Health Software – Part 1: General requirements for product safety) distinguishes between the following terms:
- HEALTH SOFTWARE
Software intended to be used specifically for maintaining or improving health of individual persons, or the delivery of care
- MEDICAL SOFTWARE
Software intended to be used specifically for incorporation into a physical medical device or intended to be a SOFTWARE MEDICAL DEVICE
- SOFTWARE MEDICAL DEVICE
Software intended to be a medical device in its own right
- MEDICAL DEVICE SOFTWARE
Software intended to be used specifically for incorporation into a physical medical device
This clarifies that medical software can be a medical device but does not have to be.
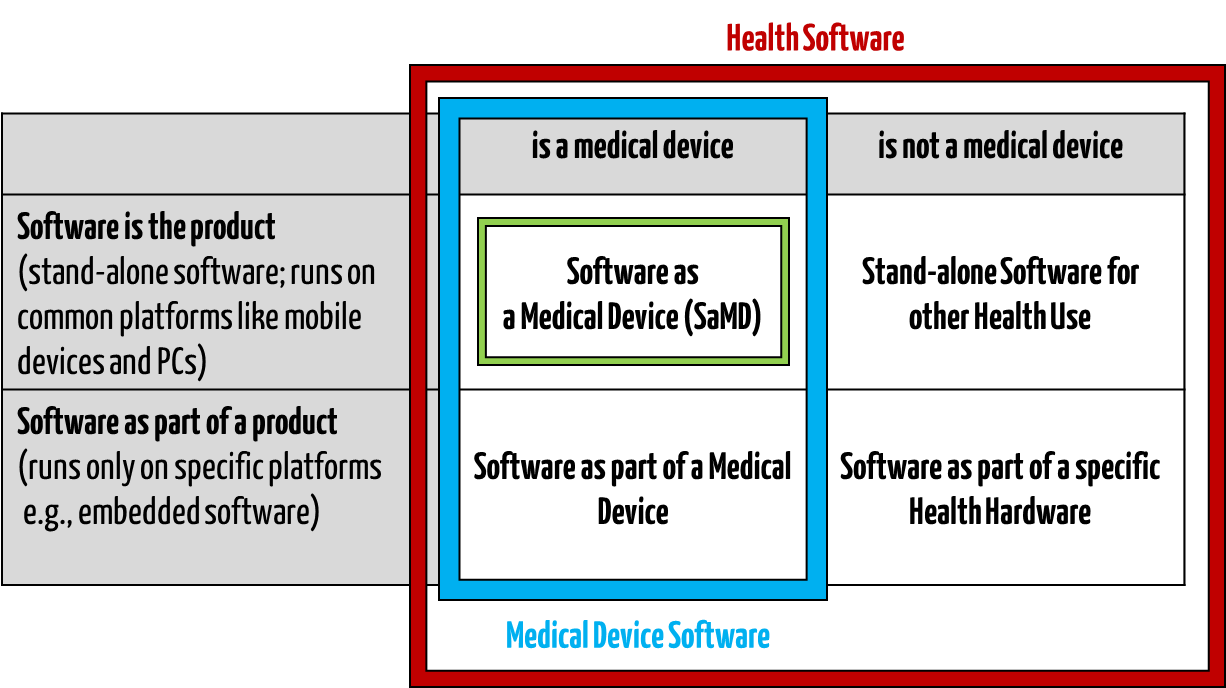
Fig. 1: Medical software includes medical device software and software as a medical device (click to enlarge).
2. Regulatory requirements
a) Medical software – a medical device?
The question often arises as to when medical software meets the definition of a medical device. You can find a further discussion on this topic in the article on the classification of software as a medical device and in the article on the qualification and classification of IVD medical device software.
b) Regulations, laws, standards
Software that is a medical device or part of a medical device must meet the regulatory requirements:
- In Europe, the medical device regulations (MDR, IVDR) are relevant. However, these only contain relatively general regulations for software, which this article presents.
- IEC 62304 defines the life cycle processes for medical device software.
- IEC 82304-1 applies to all “health software”. IEC 82304-1 also requires conformity with the requirements of IEC 62304.
- There are also MDCG guidelines, e.g., MDCG 2019-11 and MDCG 2023-4.
- The FDA sets out specific requirements in its guidance documents, including specific requirements for medical software. It also answered many questions specifically about software as a medical device in this FAQ.
3. Support for medical device manufacturers
Benefit from the support of the Johner Institute:
Contact us right away so that we can discuss the next steps. This will ensure that the “approval” is a success and that your software or devices are quickly launched on the market.
Class IIa medical devices are among the most common products on the European market. This article explains which products fall into this class, which classification rules are relevant, and what regulatory consequences this has.
Decision Support Systems are also increasingly being used in medicine. If they are medical devices, they must meet the legal requirements (e.g., the general safety and performance requirements). The hype surrounding Artificial Intelligence, in particular Machine Learning, and users such as Watson are raising hopes for the performance of Decision Support Systems. This article presents…
Details
Manufacturers who use machine learning (ML) in their medical devices or IVDs must comply with numerous regulatory requirements. This article provides an overview of the most important regulations and best practices for implementation. It saves you the trouble of researching and reading hundreds of pages and helps you prepare perfectly for your next audit.
Details
The MDR contains the Classification Rule 11. This rule is especially for software. The Rule 11 has serious implications: it bears the potential to further undermine Europe’s innovation capacity. Manufacturers should familiarize themselves with the MDCG‘s interpretation to avoid misclassifying software and to be able to follow the reasoning of notified bodies and authorities. This article…
Details
University institutions in particular regularly publish medical software as open source. This raises doubts as to whether this open-source software counts as a medical device and what regulatory and (product) liability risks are involved. This article provides a quick overview.
Details
The EU AI Act has been published. Many manufacturers of medical devices and IVD, as well as other healthcare players, are faced with the major task of understanding the 140+ pages of legal text and complying with the requirements. Note: Infringements/violations of the AI Act are punishable by a fine of up to 7% of…
Details
IEC 62304 defines safety classes so that medical device manufacturers can tailor the effort required for software documentation to the degree of harm that could be caused by a software error. This expert article helps to determine the safety classes and, if necessary, reduce them in order to minimize the effort required while still ensuring…
Details
Medical device cybersecurity is a focus not only for the FDA but also for other legislators and authorities, both in the US and other markets. This is understandable The USA has added requirements for cyber devices to the Food, Drug & Cosmetic Act (FD&C), and the FDA has published several guidance documents on cybersecurity, which…
Details
Practical guidance based on the experience of the Johner Institute, Oliver Hilgers, and Stefan Bolleininger The discussion about class I software continues to rage. This article provides guidance regarding the MDR rules for the classification of medical software.
Details
Software as medical device (SaMD) refers to (independent) standalone software that is a medical device but not part of one. It should not be confused with medical device software as defined by the EU. As a manufacturer, when do you have to qualify software as medical device, and when as medical device software? Find out…
Details
