The Medical Device Regulation (MDR) is an EU regulation that manufacturers who wish to place medical devices on the market in the EU must comply with. It also affects distributors and importers of these devices as well as notified bodies.
Regulation (EU) 2017/745 on medical devices, which is the official title of the MDR, came into force in 2017 and applies since May 26, 2021.
Tip
The Medical Device Regulation has been amended several times. You can find the consolidated version of the MDR in German and in English. These also contain the transitional periods that were extended in March 2023.
Manufacturers of in vitro diagnostics should read the article on the IVDR.
Gaining an insight into the Medical Device Regulation
Medical device manufacturers
This article on the MDR provides you with an initial overview of the Medical Device Regulation. The video linked therein presents the EU Medical Device Regulation in just a few minutes. You will also find:
- the most relevant requirements of the MDR
- the structure of the regulation
- further information, e.g., on the transitional periods
Other stakeholders
If you are not a medical device manufacturer, you should start with these pages:
At the bottom of this keyword article, you will find all other articles on the MDR.
Support with the implementation of the MDR
a) Free offers
Do you still have questions about the MDR and its implementation? You can get answers in our free micro-consulting.
Download the free starter kit. It gives you an overview of the regulatory landscape and shows you the 6 steps to “approval” of your medical device. It also contains the MDR checklist as a PDF and in DOCX format!
b) Videos and e-learning
The video training courses at our Medical Device University show you step-by-step how to create your technical documentation and QM system in a lean, fast, and MDR-compliant way. Over 100 templates and sample documents are available for download.
In this way, you create the prerequisites for approving your devices quickly and safely and launching them on the market.
c) Verification and validation
The experts at the Johner Institute can help you verify and validate your devices:
d) Consulting
Benefit from the know-how of our regulatory affairs experts to
Contact us immediately so we can clarify together how you can quickly and efficiently meet the regulatory requirements of the MDR and bring your devices safely to market.
Contact us
Chemical characterization according to ISO 10993-18 is a central component of the biocompatibility evaluation according to ISO 10993-1 and, thus, a prerequisite for the approval of medical devices. It is used to identify unknown substances in medical devices to carry out a toxicological risk assessment. This article provides an overview of
Details
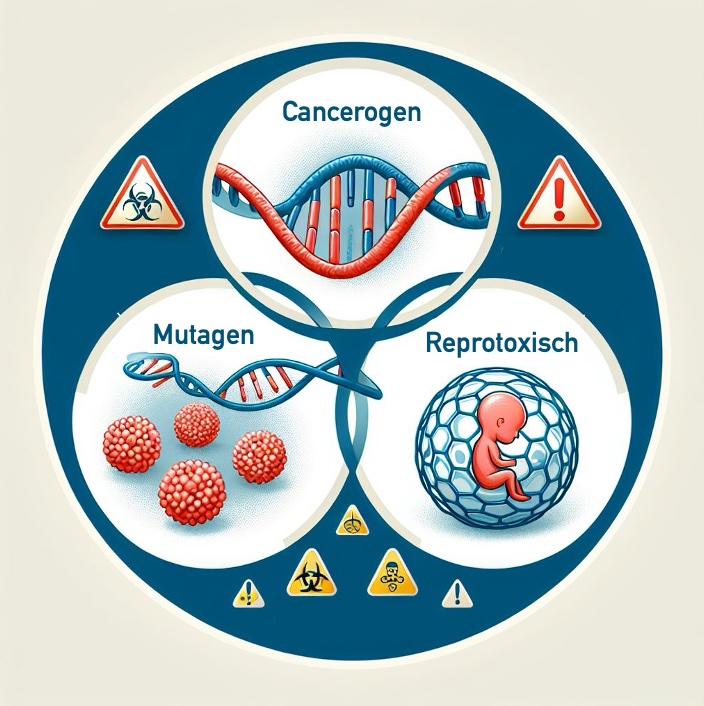
The use of CMR substances is strictly regulated. The MDR also regulates CMR substances and places strict requirements on medical device manufacturers. This article helps to fulfill these requirements.
Details
With the UDI system, the EU has introduced an obligation to identify and register medical devices that goes far beyond what is still required under the MDD. The Medical Device Regulation (MDR) even requires a UDI for standalone software. Read about what you need to prepare for here.
Details
A declaration of interest is a formal document designed to make potential conflicts of interest transparent. Individuals submit a declaration of interest to demonstrate their objectivity and independence. This article is about declarations of interest in a clinical context.
Details
The classification of whether a device is a medicinal product or a substance-based medical device has far-reaching regulatory consequences. This classification is so demanding that there are regular disputes with authorities and notified bodies, and in 2023, even the European Court of Justice had to rule on the matter. This article helps manufacturers, authorities, and…
Details
The EU regulations the MDR and IVDR set out precise requirements for importers. And they also define who is an importer. Bringing a device into the EU does not always constitute an import. And, furthermore, companies that import medical devices don’t just have to meet the requirements established for importers.
Details
Medical writers have a firm place in the ecosystem of medical device and IVD manufacturers. This article clarifies
The term “clinical validation” is also frequently used in the context of medical devices. For example, the German Federal Ministry of Education and Research (BMBF) has published a guideline on the clinical validation of innovative medical technology solutions (only available in German). The FDA also refers to clinical validation. What is clinical validation? What distinguishes…
Details
The requirements for clinical investigations to evaluate a device have increased enormously under the MDR. Learn the most important things you need to know about the regulatory pathway for clinical investigations under the MDR here.
In Article 120-123, the MDR establishes its transitional provisions, including the transition periods. However, the descriptions of these transitional provisions and transition periods are worded in a very complex manner. As a result, manufacturers are at risk of misunderstanding them and therefore not complying with regulatory requirements or incurring unnecessary costs. A flow chart in chapter 2 of this article…
Details