Phantoms in medical technology help to develop, validate, “approve” and monitor medical devices in the markets faster and more effectively.
This article describes which organizations particularly benefit from the use of these phantoms and what requirements they must meet.
1. Examples of phantoms in medical technology
Anthropomorphic phantoms are replicas of bodies that behave like real human bodies. Such phantoms are used, for example, for demonstration and training purposes during resuscitation.
In this context, anthropomorphic means that the phantoms take on or resemble the human form (“morphology”).
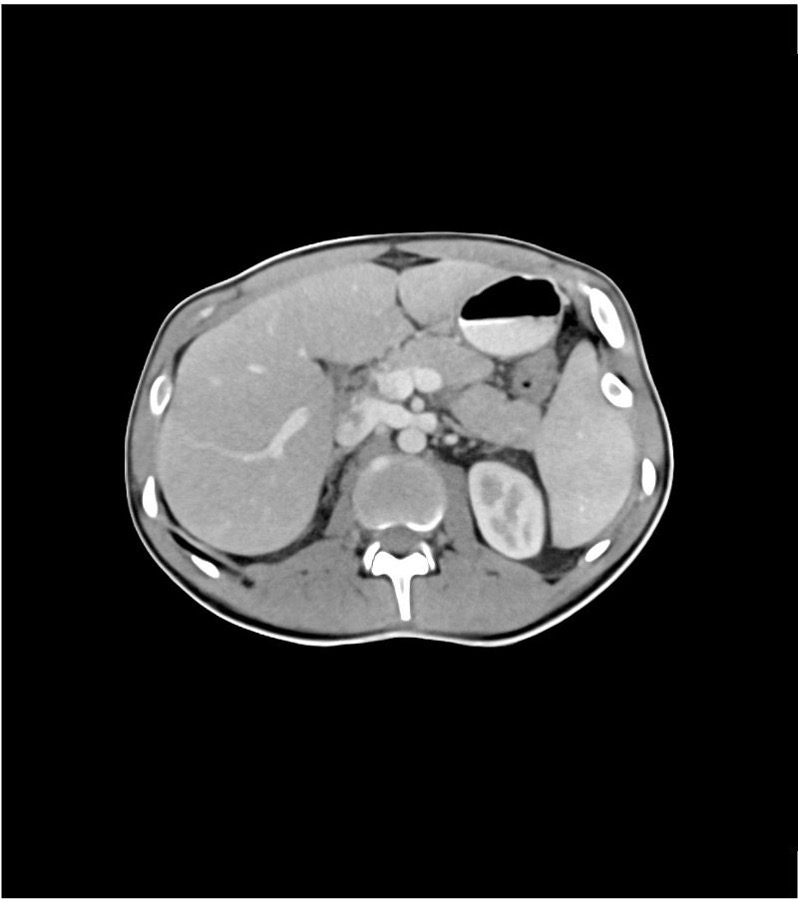
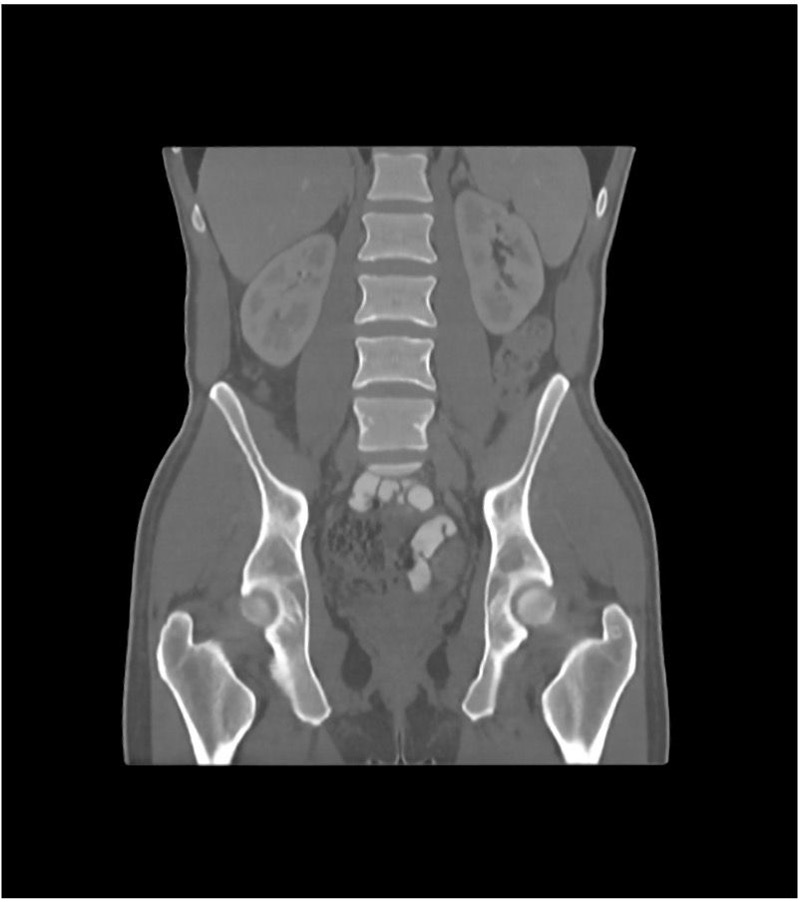
Another example is the use of anthropomorphic phantoms in radiological imaging. The phantoms behave like human tissue in a CT or magnetic resonance imaging (MRI) device, i.e., they simulate the absorption behavior of X-rays (CT) or the relaxation times of this tissue (MRI).

2. Advantages of phantoms in medical technology
Advantage 1: Faster approval
Phantoms help to reduce the number of test subjects or patients that manufacturers need to involve in clinical investigations. For example, manufacturers can use phantoms to simulate patients with rare body measurements, diseases, or tissue properties that are difficult to recruit. In addition, development loops are accelerated as manufacturers can test and validate under realistic conditions early on in the development process.
Advantage 2: Increased safety of medical devices
By using phantoms, manufacturers are able to determine the performance of their medical devices more reliably and reproduce them in a standardized manner during development and in the post-production phase.
Phantoms with defined properties also provide proof of ground truth. For example, manufacturers can create phantoms with defined geometries and “pathologies” and use them to verify their medical devices. In this way, they can ensure that the diagnostic objectives are actually achieved for the corresponding patients.
Advantage 3: Ethical arguments
The fewer patients involved in clinical investigations, the fewer patients are exposed to the associated risks e.g., by radiation or contrast agents.
Ethics committees should, therefore, at least encourage the use of phantoms in medical technology.
Advantage 4: Less effort in the post-market phase
Phantoms are also helpful when making changes to medical devices, e.g., automating verification activities and making them easier to repeat. Imaging procedures are especially susceptible to changes; at the same time, regular changes of the products are possible and often desired.
By using phantoms, performance data can be collected easily and standardized in the post-market phase.
The reproducibility of the output is not only relevant during development. Phantoms can also be used in hospitals and other health institutions for long-term quality assurance during operation.
3. Recognition of phantoms by notified bodies and authorities
Phantoms in medical technology have long been established as a special case of “computer-based modeling and simulation” (CM&S). The FDA explicitly counts CM&S as part of its regulatory strategy and has published a guidance document on the subject.
This article on computer-based modeling & simulation (German) presents further use cases and regulatory requirements in addition to the FDA guidance, including the ASME V&V 40 guideline, which is important in this context.
It is currently (only) disputed whether, which, and how much additional clinical data manufacturers must generate for clinical evaluation, particularly through clinical investigations. The extent to which phantom measurements can replace conventional verification activities is also being discussed.
Notified bodies have not positioned themselves as clearly as the FDA. However, they generally accept measurements on phantoms as the state of the art.
As a nature publication points out, phantoms in medical technology can be understood as one aspect of a larger metaverse strategy.
4. Functionality of phantoms in radiological imaging
Companies such as PhantomX produce phantoms for medical technology. They print substances that interact with radiation onto a carrier material to do this. These prints are then assembled into three-dimensional shapes.
Real and synthetic patient data serve as the basis. This enables the phantoms to simulate “real” patients and patients with desired characteristics in terms of the geometry of bodies or organs and morphological changes or pathologies.
5. Conclusion and recommendations
These organizations benefit in particular
- Manufacturers of medical devices benefit from the use of phantoms (see chapter 2). This applies in particular to
- manufacturers of medical devices for radiological imaging and
- manufacturers who work with radiological data, for example, to develop software for the analysis and diagnosis of this image data.
- Operators of medical devices should consider phantoms to verify the performance of medical devices in terms of consistency and reproducibility.
These are the next steps
We recommend that these organizations consider the opportunities that phantoms in medical technology offer.
Calculate the return on investment (ROI). Consider not only financial aspects but also the impact on regulatory safety, compliance, and patient safety.
The company PhantomX helps select and develop suitable phantoms and calculate the costs for the acquisition and use of these phantoms and, thus, the ROI.
The Johner Institute supports manufacturers in the rapid (phantom-based) worldwide approval of medical devices.