ASCA stands for Accreditation Scheme for Conformity Assessment. The procedure is intended to accelerate conformity assessments and, thus, approval procedures. However, it is not applicable to all devices or all markets.
This article explains who benefits from ASCA and how the procedure works.
1. ASCA – The basics
a) Who is affected by ASCA?
ASCA primarily affects these stakeholders:
- Medical device manufacturers whose devices fall under the IEC 60601, IEC 80601, IEC 61010, and ISO 10993 families of standards
- Test houses, which test the devices for conformity with these standards
- Accreditors (accreditation bodies) of the test houses
- Regulatory authorities, in particular the FDA
Currently, the procedure is limited to the following approval processes in the U.S.: 510(k), De Novo, Premarket Approval (PMA), and Investigational Device Exemption (IDE).
b) What is ASCA?
ASCA is a voluntary pilot program for a new accreditation system in which
- testing laboratories undergo additional qualification/accreditation and
- test reports are standardized.
The FDA trusts the test methods and outputs (primarily the ASCA Summary Test Report) of ASCA-accredited test houses and test laboratories. It does not intend to request additional information; this will expedite approval. The procedure is described below.
c) Why is ASCA needed?
The FDA criticizes the occurrence of too many incidents with medical devices. In addition to the manufacturers, the FDA has identified the inadequate quality of the test houses as one reason for this.
The authority has also recognized that medical devices are becoming increasingly complex. On the one hand, this is overtaxing the agency’s own inspectors, and on the other, it is leading to more and more queries by inspectors from experts and manufacturers.
All this ties up unnecessary resources at the FDA and delays the approval of medical devices.
d) What are the benefits of ASCA?
Thus, the ASCA program is intended to achieve the following objectives:
- Increase patient safety
- Improve patient care through more readily available medical devices
- Reduce efforts of the authority and other stakeholders (e.g., manufacturers)
- Expedite approval processes
Probably the highest benefit is for FDA investigators. They must read less and consult less frequently with internal and external experts.
ASCA-accredited testing laboratories are likely to benefit from increasing numbers of orders.
Whether the faster approval times outweigh the higher costs for manufacturers depends on the individual case.
2. Procedure
a) Qualification of the test houses
The test houses must be accredited by an accreditation body. Then they can apply to the FDA for the ASCA program. Only then are they allowed to test devices that benefit from accelerated approval (see Fig. 1).
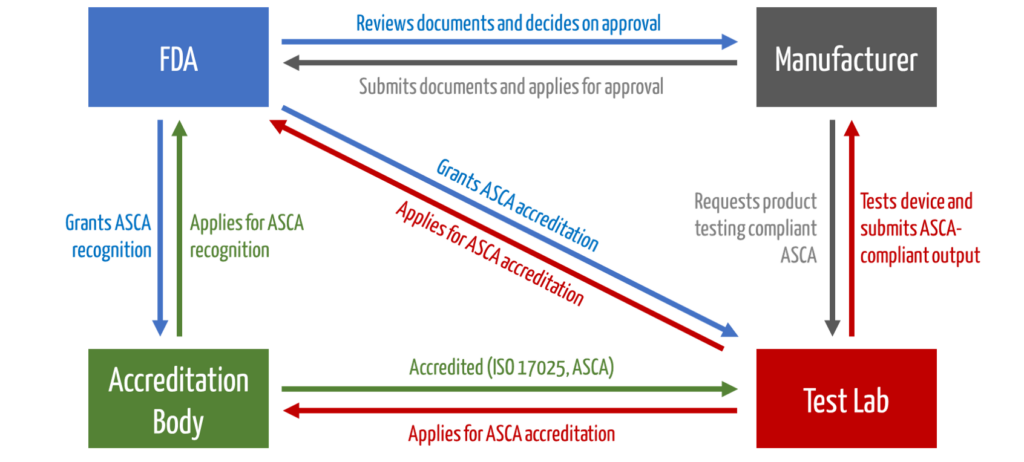
b) Approval of the devices
The manufacturers develop their devices as usual. For regulatory approval, they proceed as follows:
- Plan regulatory strategy (e.g., select and sequence target markets).
- Select a list of standards with which conformity is to be declared (This also includes standards for which ASCA should not or cannot be applied).
- Select a test laboratory (A list can be found here; in Germany, there is currently one accredited test laboratory).
- Prepare a suitable test plan with the test laboratory (this includes the necessary and applicable performance tests, which must also be performed by the test laboratory, as well as the test conditions, additions or changes to tests, and acceptance criteria according to risk acceptance).
- Test the device and summarize test results in the ASCA summary test report.
- Compile submission documents to FDA (This includes a “cover letter” with reference to ASCA tests, summary test report, ASCA declaration of conformity, and other documents). 7. Submit documents to the FDA (already, when applying via eStar, one can select that the inspection will be carried out according to ASCA, see Fig. 2).
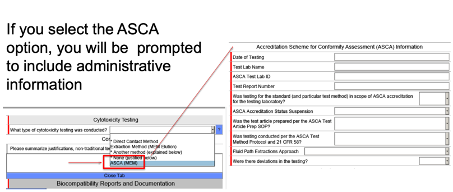
It is then the FDA’s task to review the dossier. During the approval process, the device goes through the usual phases (see Fig. 3).
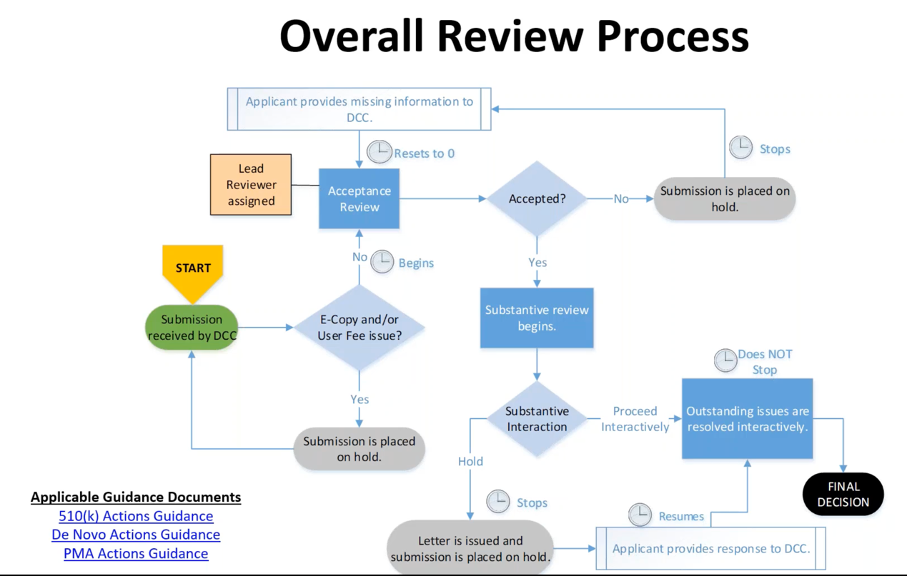
The actual time-saving concerns the steps “Substantive Interaction” and “Outstanding issues are resolved interactively.” The program should shorten these, and the iterations should even be avoided if possible.
The FDA provides extensive information on its website. Particularly relevant are the following:
3. Requirements for the ASCA test laboratories
a) ISO/IEC 17025
The QM standard ISO/IEC 17025 applies to test laboratories, and conformity with this standard is a prerequisite. ASCA specifies the requirements of this standard.
With regard to training and competence of personnel, ASCA-accredited test houses must meet the following requirements:
- Technical personnel must understand the requirements of the standards (e.g., IEC 60601-1). In particular, this includes underlying concepts such as basic safety and essential performance.
- The laboratory is required to implement a program of education and training for technical personnel.
- This program obligates training and documentation of the training and its outputs.
- It must include ongoing training (on-the-job or classroom training) that occurs at specified intervals or/and when test standards or methods are updated or newly developed.
- The testing laboratory must already specify the requirement profiles precisely in the job descriptions.
b) Overview of ASCA requirements
The requirements concern not only the competence of the personnel but also the following:
- Equipment of the testing laboratory
- Verification and validation methods
- Preparation and guidance of test plans and test instructions
- Consideration of the intended purpose and the intended use of the device in the test plans
- Preparation of test reports, especially the ASCA summary test report
- Scope and content of test reports (for example, the testing laboratory must communicate all opinions and interpretations to the manufacturers in writing, including concerns about basic safety and essential performance)
c) Additional FDA requirements
The FDA also specifies requirements for regulatory documents, specifically:
- Cover letter
- Declaration of conformity
- ASCA summary test report
- Test plan and procedure
- Acceptance criteria and outputs justifying the safety claim
4. Tips and hints
a) Have the device pre-tested
The ASCA summary test report becomes a central document that significantly reduces testing time. It is a prerequisite that the report is complete and accurate. The summary test report will also mention concerns that the test house has and mention if a test had to be repeated or if changes to the device were necessary.
Therefore, it may be useful to also have the device tested during development to avoid surprises during the ASCA inspection.
b) Create precise safety concept
Frequently encountered difficulties include insufficiently precise or even missing documentation of the safety concepts or the system architecture.
A blanket statement such as “The device must meet the requirements of IEC 60601-1” will no longer suffice. Notified bodies in Europe are already taking a closer look today, and testing laboratories must do the same.
This means that manufacturers should specify the safety requirements and safety goals regarding single-fault safety during the design phase and then benefit from the safety concept as a guideline to build safe systems. The basic standard for functional safety IEC 61508 can provide informative assistance.
Read more about systems engineering.
c) Specify acceptance criteria
Test plans should specify appropriate test methods for performance testing with accurate acceptance criteria. Both manufacturers and test laboratories should note that systems have dynamic behavior. This is often not tested.
Test labs are then able to create an appropriate test plan more quickly and define an appropriate test setup with the manufacturer.
Other important information for the test laboratory is:
- Intended purpose/intended use, including intended use environment and intended user
- Comprehensive product description
- Systematic derivation of essential performance characteristics
- Discussion of the special properties of the technologies used
- Information on labeling, especially with regard to the intended purpose and use under various circumstances
- Evidence of performance testing, such as test reports and clinical data
- Derivation of limit values and acceptance criteria
- Description of any changes to tests or additional tests that are necessary
This information can either be included in the product description, in the architecture document, or in individual documents. There is no default for the structure. However, this is not new, because notified bodies and the FDA already require all this information anyway.
d) Consider costs
The FDA estimates the additional effort of the test laboratories to prepare the reports to be about 47 hours, i.e., about one working week. This is likely to entail costs in the order of EUR 10,000.
5. Summary and conclusion
a) Possible disadvantages
Test laboratories have a higher overhead, which drives up costs and may prevent smaller test laboratories from participating in the program. This could result in limited testing capacity.
The test labs will pass the cost on to the manufacturers. One possible consequence is that companies that cannot afford the cost will not be able to benefit from FDA’s expedited procedures. If so, the FDA will not achieve its objective of getting innovative medical devices to market faster.
b) Possible advantages
Precise requirements also have advantages:
- They save all parties involved (manufacturers, test houses, approval bodies) unnecessary discussions.
- Manufacturers know early on what to expect. Accordingly, they can ensure conformity at an early stage, which saves expensive rework and iterations.
- This makes testing and approval procedures faster and easier to plan.
6. Updates on the ASCA program
In September 2024, the FDA published an update to the draft guidance Basic Safety and Essential Performance of Medical Electrical Equipment, Medical Electrical Systems, and Laboratory Medical Equipment – Standards Specific Information for the Accreditation Scheme for Conformity Assessment (ASCA) Program.
Read the most important information at a glance:
a) From pilot project to full program
The ASCA program has developed from a pilot project to a full program, and manufacturers can voluntarily use an ASCA-accredited testing laboratory. Only testing laboratories with accreditation from a US agency may participate, while in Germany, only testing laboratories that also have international accreditation may participate. Test reports from laboratories accredited by the German accreditation body (DAkkS) will continue to be recognized by the FDA.
Here, you will find a list of currently accredited testing laboratories.
ASCA testing laboratories are regularly audited according to extended requirements beyond ISO/IEC 17025 to ensure their competence.
b) Extended collaboration and testing standards
Testing laboratories will work more closely with manufacturers to create test plans. The focus here is on the two topics of electromagnetic compatibility (EMC) and essential performance. The updated draft guidance emphasizes the essential performance of a device, which must be maintained to avoid risks to patients or users. Test laboratories must understand this concept and work closely with manufacturers to ensure compliance with these requirements. Test reports must also indicate any problems that occurred during the test.
In the future, manufacturers can assume that ASCA testing laboratories will not only formally check the risk management file but will also evaluate its consistency in terms of content and, if necessary, plan additional tests. It is hoped that the notified bodies will recognize the assessments of ASCA testing laboratories. However, we currently often see notified bodies deviate from laboratory assessments and record their own deviations.
c) Why these changes are important
The focus of the testing laboratories has so far been strongly on basic safety. With the ASCA program, the FDA is shifting the focus toward the functional safety of electrically operated medical devices (including IVD). This means that manufacturers must fully identify the risks and describe the functional safety in the architecture or a safety concept in an understandable manner.
The requirements for demonstrating the safety of medical devices will become stricter in the future, independently of the ASCA program. A working group we belong to is currently developing the technical report IEC TR 60601-4-9, “Medical electrical equipment – Part 4-9: Functional safety for essential performance – Guide and interpretation.” The report is currently in draft form and is scheduled for publication in 2025. By then (at the latest), testing laboratories will be more intensively checking medical devices’ functional safety.
d) FDA revokes ASCA accreditation for several laboratories
Update September 2025: In September 2025, the FDA announced that several ASCA-accredited testing laboratories had their accreditation revoked. These laboratories are now marked with the status “FDA Initiated Withdrawal” in the ASCA-Accredited Testing Laboratory Database.
Background: During audits of ASCA-accredited testing laboratories, the FDA found that some laboratories did not meet the requirements of Section 514(d) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the associated ASCA program guidelines. In some cases, serious concerns were identified regarding the data integrity of premarket submissions.
As of October 2025, there are no longer any active ASCA-accredited testing laboratories in Germany.The only German laboratory (UL International Germany GmbH, Neu Isenburg) voluntarily withdrew its accreditation. There are currently ten ASCA-accredited laboratories in the EU: five in Italy, two in the United Kingdom (non-EU), and one each in Poland, Spain, and Sweden.
e) New opportunities for IVD manufacturers through IEC 61326-2-6
Update July 2025: In July 2025, the FDA fully recognized the new edition of the IEC 61326-2-6 Edition 4 standard. This standard regulates the electromagnetic compatibility (EMC) of in vitro diagnostic (IVD) medical devices.
Significance:
- The standard is part of the IEC 61010 family and therefore falls within the scope of the ASCA Basic Safety and Essential Performance Guidance.
- This expands the opportunities for IVD manufacturers to participate in the ASCA program.
Benefit from the Johner Institute’s help to pass all approval procedures quickly and with minimal effort, with precise documentation and safe medical devices.
We support you in preparing your documents (e.g., safety concept, risk analysis, defining essential performance characteristics, test plans, test strategy) or taking over this work entirely.
Our experts also assist you in reviewing all documents, such as the architecture (compliant with ISH1, with functional safety methods) and all other “submission documents.”
Change history:
- 2025-10-21: Laboratory accreditation withdrawal (Sept. 2025), new IEC 61326-2-6 standard for IVD (July 2025), Germany without ASCA laboratory, current EU laboratory overview, clarification on fees
- 2024-11-25: Chapter 6 added with updates on the ASCA program
- 2023-06-20: Article initially created

The gold standard is testing acc. to the CB-scheme from the IECEE and receiving a CB-report and certificate. Like the FDA requires in the ASCA-program, the test laboratories are accredited and controlled within the CB-scheme. Why did the FDA create a new system? Have they trust issues with the CB-scheme?
Greetings
Andreas Meschberger
Dear Mr. Meschberger,
That is a good question. When reviewing the test reports, the FDA often finds errors, particularly in the assessment of safety. There are obviously too many incidents involving medical devices, even though medical devices are reviewed by three bodies. One source is the lack of knowledge in the test houses. This is also my experience. The problem is not the electrical hazards, but the issues such as single fault safety, risk analysis and essential performance characteristics. Another goal of the FDA is to reduce the lead time for testing and save internal resources in the process. ASCA test reports are reviewed in a shorter time.
Best regards, Mario Klessascheck