The standard ISO 15223-1, regulates the symbols that manufacturers are permitted to/must use for labeling medical devices.
In January 2022, the EU Commission harmonized EN ISO 15223-1 as one of relatively few standards under the MDR and added it to the list of harmonized standards. This alone makes it clear how relevant labeling has become.
Read this article to find out what ISO 15223-1:2021 describes and requires and where you can obtain the symbols.
Update: The amended version of 2024 is available as a draft (see below).
Regulatory background
The MDR obliges manufacturers to provide information on the labels (see Annex I, Section 23.2). This includes:
- Lot or serial number
- Shelf life and/or date of manufacture
- Storage conditions
- The fact that the device is a medical device
- Contains medicinal products
- Contains tissues or derivatives of human or animal origin
- Contains carcinogenic, mutagenic, reprotoxic, or endocrine disrupting substances
- Has been sterilized
- May or may not be reused
- If applicable, the EU representative
Manufacturers usually use symbols to communicate this information clearly and in a minimum of space. These symbols, in turn, are defined by standards such as ISO 15223-1. Additional product or process-specific standards, such as IEC 60601-1, have requirements for the symbols to be used, which are not (yet?) harmonized.
Due to national requirements of the EU member states for the accompanying information, it is necessary to provide all information required for regulatory purposes in accordance with the MDR in a recognized language (usually the official language(s)). This means that information must be translated into text form – fortunately, the use of recognized symbols saves manufacturers this effort, at least for labeling. However, the instructions for use must still be translated if necessary.
About ISO 15223-1
Structure of the standard
At 74 pages, ISO 15223-1:2021 and its German equivalent, DIN EN ISO 15223-1:2022, is not a short standard, but it is quite straightforward thanks to the numerous tables:
- 22 pages of it are devoted to Annexes ZA and ZB, which establish the mapping between the requirements of the EU Medical Device Regulations (MDR and IVDR).
- The following 10 pages contain the foreword, introduction, definitions, and a few normative requirements, such as the size of the symbols derived from the intended function. Here, ISO 15223-1 also requires the symbols to be used on the medical device, on the packaging, or in the documentation provided (e.g., instructions for use).
- This is followed by 25 pages of tables presenting the symbols and their meaning.
- A further 9 pages use examples to explain the possible uses.
- The rest of the document consists of cover pages, and directories.
New version 2021
The current harmonized version EN ISO 15223-1:2021 contains some new features and 25 new symbols.
The following changes have been made compared to DIN EN ISO 15223-1:2017-04
- Inclusion of 25 new symbols
- Update of the definitions taking into account DIN EN ISO 20417, DIN EN ISO 14971, and DIN EN ISO 13485
- Revision of the requirements for using symbols (4.2) (among other things, the first paragraph with a similar meaning has been completely reworded. In addition, the manufacturer “must” now specify the appropriate size of the symbol instead of just “shall”).
- Extension of the examples in informative Annex A to notes and examples for the use of symbols, including the joint use of several symbols
- Addition to the informative annexes on the relationship between this European Standard and the general safety and performance requirements of Regulation (EU) 2017/745 on Medical Devices which is to be covered and the relationship between this European Standard and the general safety and performance requirements of Regulation (EU) 2017/746 on In Vitro Diagnostic Medical Devices which is to be covered
- Change the title to “Symbols for use in the context of information to be provided by the manufacturer”
- Editorial revision
According to Annex I, Article 23.1 h) of the Medical Device Regulation MDR (2017/745), the corresponding new symbols must be used. Due to the harmonization of EN ISO 15223-1:2021, it is not always necessary to explain the symbols listed in the standard in the instructions for use. However, this only applies if it does not lead to higher risks from the device. Manufacturers should determine whether this is the case in usability studies.
However, if the device is intended for lay users, the symbols must always be explained. Manufacturers cannot assume that laypersons will understand symbols without an explanation. Time will tell whether the numerous new and very detailed symbols can be used in practice without a laser printer – especially when you consider that the implantation ID card, for which some symbols were probably specially created, is supposed to be the size of a credit card.
The additional symbols are as follows:
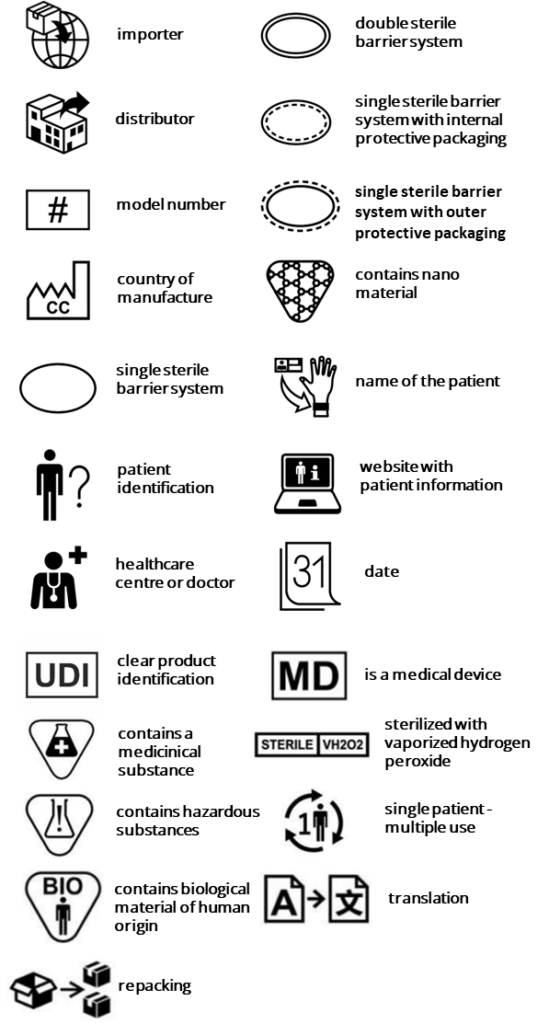
Amendment 2024
At the European Commission’s request, ISO 15223-1 is to be amended. To avoid confusion with Ecuador (ISO 3166 ALPHA-2 country code EC), the symbol for the EU representative must be changed from EC to EU-REP.
Even if Ecuador is not obligated to display the local representative on the label, counteracting this potential misleading effect is, of course, laudable. At currently 10 pages (in draft form), the amendment is very short and, at a good €40, not extremely expensive.
But: per relevant letter (U instead of C), this is probably the most expensive standard of all time. This adaptation is irrelevant to safety, so it is unclear why an immediate change is required and why manufacturers should pay for it. After all, the country abbreviation EC for Ecuador dates back to the last millennium. Especially since many manufacturers are hoping for symbols for placing systems on the market in accordance with Article 22 of the MDR or for the indication that rapid tests are not intended for self-testing (Article 20.2 r) in Annex I of the IVDR) in order to avoid text on the label.
We, therefore, advise against purchasing the modification for the moment. You can find outwhere to get the symbol below. However, please remember to update your technical documentation accordingly.
Example for the definition of symbols
ISO 15223-1:2021 determines the requirements for the presentation and use of symbols in tabular form. The tables contain the columns
- Reference number and graphic
- Symbol title
- Symbol description
- Requirements
- Notes
- Restriction of use
- ISO/IEC symbol number and registration date
Using the “manufacturer” symbol as an example, this looks as follows:
| Reference number and graphic | Title | Description | Requirements | Notes | Restriction of use | ISO/IEC symbol number and registration date | |
5.1.1 | Manufacturer | Shows the manufacturer of the medical device | The symbol must appear together with the name and address of the manufacturer close to the symbol. | NOTE 1 This symbol is used to indicate information required in Europe and may be required by other competent authorities […] | – | ISO 7000-3082 2011-10-02082 2011-10-02 |
DIN ISO 7000 is entitled “Graphic symbols on equipment.” The symbols defined therein are not limited to the area of medical devices.
The ISO website lists all symbols:
The ISO offers these for purchase. A free publication from MedTech Europe contains most of the symbols.
Change history
- 2024-08-22: Section amendment 2024 added, references to ISO 980 removed, reference to national specifications added, wording adapted to ISO 15223-1:2021
- 2022-08-17: Notes on the 2021 version added
- 2021-11-30: Publication from MedTech Europe added
- 2021-08-17: New version section updated



Hello
We are company of medical device type II (sterelised needle) .Level of packagings are as following:
1 ) blister (direct packaging)
2) Dispenser 30 or 100 units
3) Shelf (about 1400 dispensers)
4) Shipper same as shelf (protective carton)
1)What is the alternative at blister packaging level , if we not indicate the manufacturer details : IFU, UDI etc is allow instead ?
2) same questions on Shipper level : what is the laternative ?
In Europe,US, Canada, turkie ?
3) What are the symbol that are mandatory according with packaging level?
Dear Nathalie,
the labeling on the sterile barrier system (SBS) – I assume in your case blister level, as these maintain the sterility of your device – is regulated either by the MDR (in Europe and also Türkiye) or by the recognized consensus standard ISO 11607-1 (EU, Türkiye, USA and Canada). In any case, the regulations require the manufacturer details directly on the SBS, there is no alternative.
Or are your devices not sold individually but only in the dispensers as the point of use? Then this dispenser could be considered as the outer protective packaging of your SBS and carry all required information.
The shipping packaging is only intended for transport and thus is not considered an additional packaging level, and as such is not required to fulfill any regulatory requirements. However, in certain cases (e.g. customs) a clear indication of the manufacturer is required to make the shipment traceable.
The information required on the packaging can be found in the MDR and 21 CFR part 801 as well as ISO 11607-1, the corresponding symbols in ISO 15223-1.
Let us know if we should discuss this in more detail in a short workshop, based specifically on your own device.
Kind regards
Christopher Seib