Brexit was completed in January 2021. What was a cause for celebration for some means an additional burden for many – including medical device manufacturers.
For manufacturers, it is important to understand which regulatory requirements they will have to fulfill and which transition periods they will benefit from if they want to continue selling their devices in the UK.
1. Regulatory requirements after Brexit
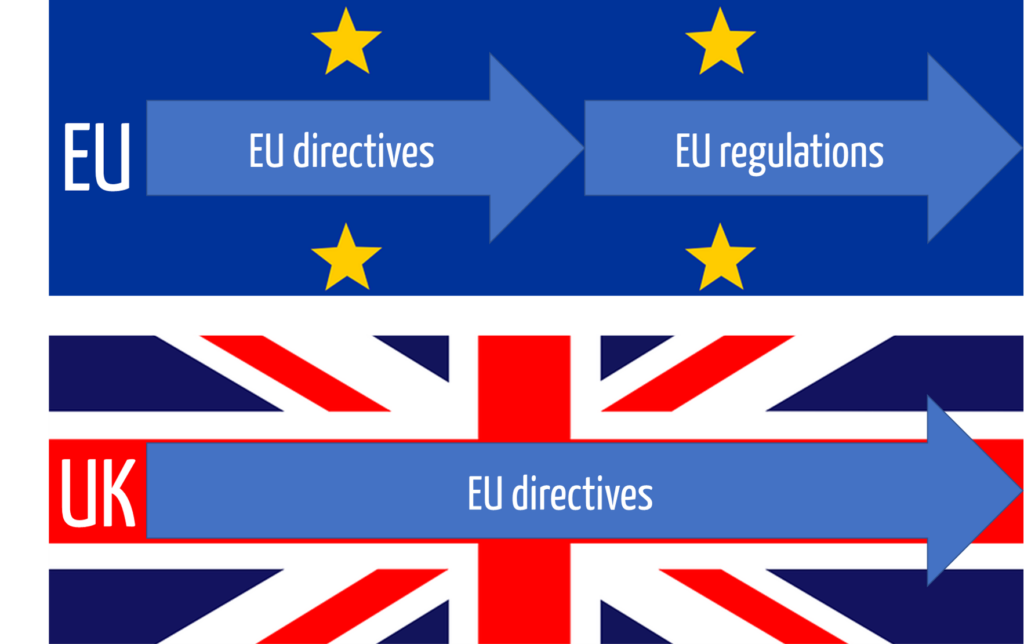
a) UK law applies and therefore the EU directives!
The transition period during which EU law was still applied in the United Kingdom ended on December 31, 2020. Since January 1, 2021, the law of the United Kingdom applies, although it continues to be based on old EU law:
The UK Medical Devices Regulations 2002 apply to medical devices, which in turn continue to reference the (old) EU directives, i.e.:
- Medical Device Directive MDD (93/42/EEC)
- Active Implantable Medical Device Directive AIMDD (90/385/EEC)
- In vitro Diagnostics Directive IVDD (98/79/EEC)
Manufacturers who have just switched to the MDR would, therefore, have to do a roll backward. The good news, however, is that the MDR requirements are rather the superset of the requirements formulated by the EU directives.
b) Conformity assessment procedures and CE marking remain valid – for now
With the EU directives, the requirements for conformity assessment procedures and CE marking remain in place in the UK.
Initially, the UK accepted the placing on the market with CE marking only until June 30, 2023. In the meantime, however, these transition periods have been extended (see chapter 2). Declarations of conformity according to the old EU directives as well as the MDR and IVDR, will be recognized. This means that manufacturers do not have to undergo an additional conformity assessment procedure during this period.
After the transition periods, manufacturers must undergo a UK-specific conformity assessment procedure. However, this does not result in a CE marking, but the UKCA marking, i.e., marking with the UK Conformity Assessed Mark.
c) The notified bodies will be followed by the UK Approved Bodies
For UKCA marking, manufacturers must involve a UK Approved Body. This corresponds to the notified bodies because the UK conformity assessment procedure is still based on the EU directives, at least until the UK enacts other requirements. The foundation for a fundamental revision of the current law has been laid by the Medicines and Medical Devices Bill, passed in February 2021.
However, the UK is not free to change or even increase these specifications at will. The Brexit deal sets limits to this because the largely free movement of goods is only allowed if neither side uses regulation to close off the respective market.
Because the UK requirements currently correspond to the EU directives, manufacturers of class I devices do not have to involve a UK Approved Body in the conformity assessment procedure.
d) New is the obligation to register
However, manufacturers importing devices into the UK must register them with the UK’s Medicines and Healthcare products Regulatory Agency (MHRA).
The manufacturer is not allowed to carry out the registration. Instead, the UK Responsible Person is responsible for this.
We are your reliable authorized representative for medical devices in the United Kingdom, ensuring your market access.
More information on this can also be found below.
Read more about medical device registration on the MHRA’s pages.
The Regulating Medical Devices in the UK page is also helpful.
e) Summary: 3 steps to approval in the UK
EU manufacturers who wish to market their medical devices in the UK must proceed as follows:
- Find and register a UK Responsible Person.
- Register their own devices through this UK Responsible Person.
- Place the devices on the market in the UK.
2. Transition periods for the recognition of the CE marking
CE-marked medical devices can currently continue to be marketed in the UK. Accordingly, no additional UKCA marking is required. The following transition periods apply:
| Applicable EU regulation | Transition period |
| Medical devices with valid CE marking under the MDD or AIMDD | Maximum June 30, 2028 (or until certificate expires) |
| In vitro diagnostic medical devices with valid CE marking under the IVDD | Maximum June 30, 2030 (or until certificate expires) |
| Medical devices (including custom-made devices) and in vitro diagnostic devices with valid CE marking under the MDR or IVDR | Maximum June 30, 2030 |
With the end of the transition period, the MHRA insists on going through the conformity assessment procedures under UK law with the involvement of a UK Approved Body.
Meanwhile, the MHRA is making efforts to recognize devices with existing approvals in other countries, even beyond the above-mentioned transition periods. Based on the current situation, manufacturers with approvals in Australia, the EU, Canada, or the USA would benefit from this.
3. Required roles
a) UK Responsible Person
With Brexit, a UK Responsible Person has become mandatory. This person must be resident in the UK and registered with the MHRA. The UK Responsible Person must perform the following activities comparable to the authorized representative according to MDR and IVDR:
- Register devices
- Act as the primary point of contact for the MHRA
- Report information from the field to the manufacturer, including complaints and reports from users, patients, and healthcare facilities (especially regarding potential serious incidents)
- Assist with the implementation of field safety corrective actions and other corrective and preventive actions
- Verify that the manufacturer has gone through a conformity assessment procedure
- Make sure the declaration of conformity has been created and keep it available
- Make sure the technical documentation has been created and keep it available
- Provide the MHRA with a list of importers
If the UK Responsible Person suspects that the manufacturer is not fulfilling its legal obligations, it must resign its mandate and inform the MHRA and, if appropriate, the UK Approved Body.
We are your reliable authorized representative for medical devices in the United Kingdom, ensuring your market access.
b) Importers and distributors
Importers must inform the UK Responsible Person about their activities. They can also assume this role at the same time. Distributors have no additional obligations to fulfill.
c) UK Approved Body
After the transition periods have expired, all medical device manufacturers who wish to market their devices in the UK must involve a UK Approved Body – at least if their devices fall into classes I*, IIa, IIb, or III.
Currently, there are only four accredited companies, according to the UK government’s website: SGS, BSI, DEKRA, and UL.
This list has now been extended to include the German TÜV SÜD, among others.
4. Further requirements
a) General safety and performance requirements
Because the regulatory requirements for medical devices marketed in the UK continue to be formulated in EU directives, there are no different or additional essential requirements. This will change when the new legislation is introduced (probably in 2025). For an outlook, see this document from the MHRA.
In the meantime, MHRA has started to formulate its thoughts on human factors engineering. These are somewhat more closely aligned with the FDA’s requirements than with those of the old IEC 62366. On the other hand, manufacturers who follow IEC 62366-1 should also meet the MHRA’s usability requirements.
b) UDI
Since the old directives did not require a UDI, this obligation does not apply in the UK for now. Of course, there is nothing to prevent manufacturers from placing devices on the market in the UK with a UDI.
c) Quality management systems
The requirements of the MHRA for the QM system do not differ from the corresponding requirements in the EU. Here, too, ISO 13485:2016 or the requirements of Annex II of the MDD, for example, are decisive.
d) Post-market surveillance & vigilance
The requirements for post-market surveillance and, in particular, vigilance have always been specific to individual countries, e.g., regarding the format, deadlines, and recipients of vigilance reports.
Similar to the introduction of the MDR, despite transition periods, post-market surveillance (PMS) requirements will have to be applied even before the new legislation comes into force. This decision took effect on June 16, 2025, with the addition of Part 4A to the UK Medical Devices Regulations. The MHRA provides extensive guidance on PMS and further information on its website.
5. Special case Northern Ireland
The situation is particularly complicated for devices that are to be marketed in Northern Ireland. These are not subject to British law but to EU law. This means that the MDR and IVDR will apply in Northern Ireland from May 26, 2021, and May 26, 2022, respectively!
Thus, according to the current status in Northern Ireland, the obligation for CE marking remains even after the transition periods. However, a UK Responsible Person is still required who is responsible for the registration of the devices in the MHRA database, among other things.
An exception to the obligation to have a UK Responsible Person applies to class I devices, custom-made devices, and other IVDs that have already been registered in the EU.
Another special regulation concerns the label: If a UK Approved Body is involved in the conformity assessment, then the UKNI label is required in addition to the CE marking. However, the combination of both labels is only intended for the Northern Ireland market and is mandatory in this case. Within the EU, only the CE marking may be used. Additional application of the UKNI label when placing on the market in, e.g., Germany, is not permitted.
In addition to the CE marking, device manufacturers also need to apply the UKNI marking if they choose to use a UK Notified Body for mandatory third-party conformity assessment. Device manufacturers must never apply the UKNI marking on its own – it must always accompany a CE marking. To place goods on the EU market, manufacturers must use the CE marking on its own, without the UKNI marking. Goods bearing the “CE & UKNI” marking will not be accepted on the EU market.
MHRA website
Read more about the special arrangements for Northern Ireland and the UKNI marking in communications from the UK government (PDF).
6. Requirements for UK manufacturers
For British manufacturers who want to place devices on the market in the EU, European regulations such as the MDR apply. This means that they will need an EU-notified body and an EU-authorized representative from January 1, 2021. Devices that have already been placed on the market in the EU before January 1, 2021 may continue to be made available and put into service there.
If UK manufacturers wish to market their devices in Northern Ireland, they will need an EU-authorized representative based in Northern Ireland.
7. Conclusion
As a result of Brexit, there are no winners, at least in the world of medical device manufacturers, but (almost) only losers.
Loser 1: British patients
If MDR and IVDR were to fulfill their promise to increase the safety, performance, and benefits of medical devices compared to the previous directives, then UK patients would lose out. This is because, in the UK, for now, devices only have to meet the “old” requirements of the EU directives.
Loser 2: The British healthcare system
Placing on the market becomes more burdensome for EU manufacturers.
- An additional role, the UK Responsible Person, must be created.
- When the transition periods expire, EU manufacturers will have to go through a second conformity assessment procedure.
- They will then need (depending on the class) a UK Approved Body in addition to the notified bodies.
This additional effort must be paid for – ultimately by the UK healthcare system. Or the manufacturers decide to no longer market their devices in the UK. In that case, they will be missing in the British healthcare system.
Loser 3: The EU manufacturers
For the EU manufacturers, this means the additional effort described above for registration, the additional conformity assessment procedure, and the additionally required UK Approved Body.
Loser 4: The authorities (EU and UK)
No matter if customs authorities, MHRA, or European authorities are responsible for market surveillance: All of them have an additional bureaucratic effort that does not bring additional safety. On the contrary: redundant or additional and unnecessary tasks keep the authorities from fulfilling their actual duty. A duty that is already obviously more than challenging.
Summary
“Take back control” was one of the slogans of the Brexit supporters. The result is that Great Britain is working with outdated regulations. That these are EU regulations seems like a tragicomic twist of history.
Good medical devices are good medical devices. Therefore, the objective should be to create uniform regulations worldwide. Unfortunately, Brexit was a step in the opposite direction. So was the MDR with the concept of Common Specifications and the lack of harmonized standards to date. It’s a shame.
Benefit from Johner Institute’s help to meet the UK requirements for medical devices in compliance with the law and with minimal effort.
We support you in registering your devices with the MHRA and take on the UK Responsible Person role.
Our experts will also assist you in preparing and reviewing all necessary documentation.
Change history:
- 2025-06-20: Information about the introduction of the new PMS requirements in chapter 4 d) added
- 2025-04-07: Note added to chapters 1 and 3
- 2024-10-15: Addition regarding the planned recognition of international approvals
- 2023-12-04: TÜV SÜD added
- 2023-06-22: Article substantially revised with reference to the new transition periods and planned legislation