Many laws and standards require validation. However, these regulations define the term differently in various contexts.
Content
This article provides a quick overview and links to practical tips in further articles:
- Basics
- Validation of medical devices
- Validation of tools and computerized systems
- Validation of processes
- Support
1. Basics
a) Definition
ISO 9000 defines the term validation as follows:
Definition (1)
Confirmation by objective evidence that the requirements for a specific application or intended purpose have been met
Source: ISO 9000
As the term requirement is interpreted differently, this definition is helpful:
Definition (2)
Confirmation by objective means of whether specified users achieve the specified objectives in the specified context of use
Source: Johner Institute, based on IEC 62366-1
b) What can be validated
The definitions do not determine which objects are validated. In the context of medical devices, these are, for example
- medical devices
- components of these devices
- processes for developing, producing, and monitoring these devices
- tools and computerized systems
2. Validation of medical devices
a) An activity in the development process
For medical devices, validation occurs at the end of development (see Fig. 1). This applies not only to the V-model but to every development process.
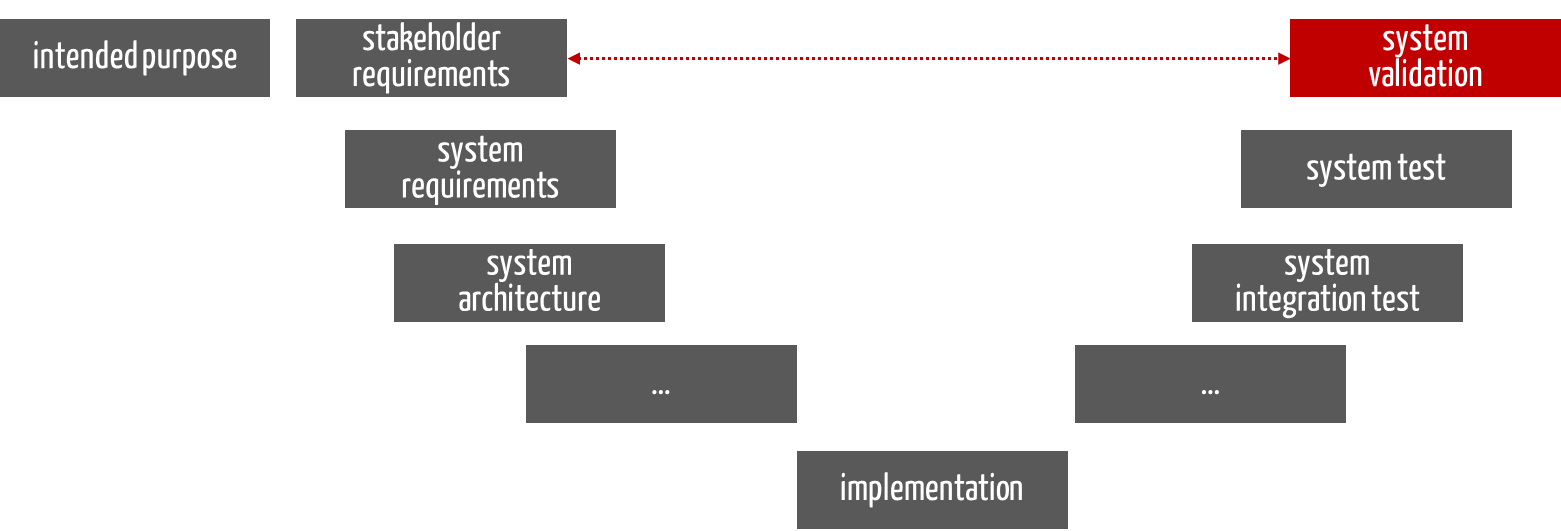
Fig. 1: Validation takes place at the end of the development process.
This is referred to as design validation.
It must ensure that the stakeholders’ requirements are met and that the users achieve the intended purpose (see dashed red arrow in Fig. 1).
b) Methods
The most important methods in the validation of medical devices are clinical evaluation and usability validation, e.g., in the form of a summative evaluation or usability tests.
In the V&V of IVDs, the performance evaluation corresponds to the clinical evaluation.
Some manufacturers also include tests in test laboratories, such as biocompatibility tests (e.g., cytotoxicity tests) and electrical safety tests in validation. But these tend to be verification activities.
Caution!
The term “validation” is also used in the context of components, such as machine learning libraries. However, these tend to be verification activities in the regulatory sense.
c) Regulatory requirements
All important standards and laws require the validation of medical devices. These include:
- MDR: in Annex II
- ISO 13485: in Chapter 7.3.7 (“Design and development validation”)
- FDA: in 21 CFR part 820 (which is largely replaced by ISO 13485)
- IEC 82304: in Chapter 6 for health software
ISO 13485 counts clinical evaluation as part of validation.
3. Validation of tools and computerized systems
In several instances, ISO 13485 requires manufacturers to validate their infrastructure and tools. Chapter 4.1.6, for example, obliges manufacturers to perform Computerized Systems Validation (CSV). Standards such as ISO 80002-2 and AMII TIR 36 assist with this.
This article describes the exact requirements of the standard for tool validation.
4. Validation of processes
Laws and standards such as the MDR, IVDR, and ISO 13485 also oblige manufacturers to validate processes. All processes that have an impact on the conformity of the devices must be validated. These are not only development and production processes but also processes for dealing with customer feedback and post-market surveillance.
5. Support
Do you still have questions about the validation of medical devices, computerized systems, and processes? Our free micro-consulting will provide you with answers.
You will learn how to validate processes in the internal auditor seminar and the certified auditor course. The computerized systems validation seminar provides you with all the knowledge you need to carry out CSV yourself.
A usability validation in the Johner Institute’s usability labs ensures the conformity and usability of your devices.
Contact us here if you would like support in determining and verifying all relevant requirements quickly, easily, and in compliance with the law. We will help you avoid hassle and delays in the approval process and quickly get your devices onto the market.
