The post-market phase is the phase following the placement of medical devices on the market. ISO 14971 also refers to the post-production phase after development and production.
Content
This page provides a quick overview of the articles on the post-market phase:
- Life-cycle of medical devices
- Regulatory requirements
- Activities in the post-market phase
- Responsible roles
- Interaction of the post-market phase with risk management
- Support
1. Life-cycle of medical devices
The post-market or post-production phase comprises all activities after production until the decommissioning/decommissioning of the devices, at the latest, at the end of their service life. These activities also include use and maintenance.
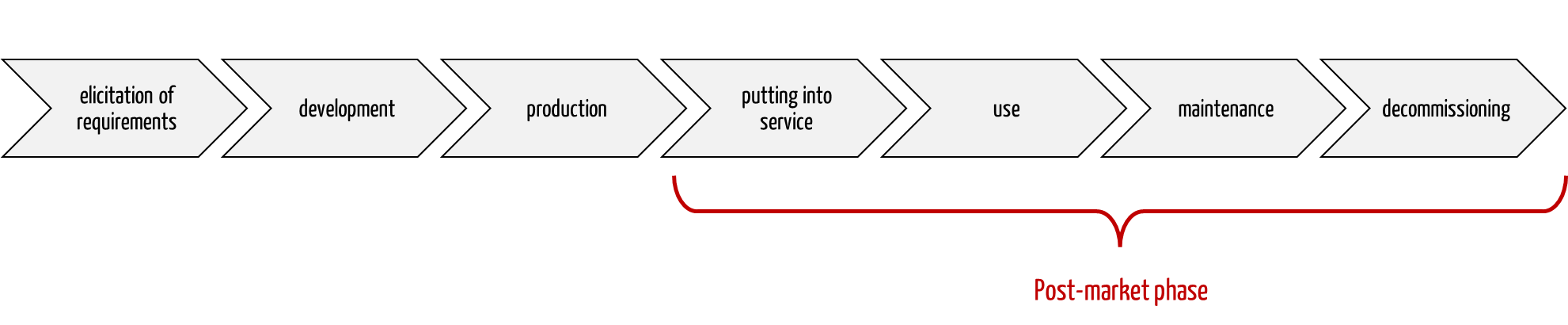
Fig. 1: The post-market phase is an important part of the product life cycle.
2. Regulatory requirements for the post-market phase
The international regulatory requirements for the post-market phase are increasing. The EU MDR and IVDR regulations are evidence of this. In addition, there are national regulations, such as 21 CFR part 822 on post-market surveillance, and national laws and regulations on vigilance, such as the German law MPAIMV.
There are also guidelines, such as MDCG 2022-21 and MDCG 2020-6, and laws that apply to all devices, such as the Product Liability Act, which also requires post-market activities such as monitoring.
3. Activities in the post-market phase
a) Post-market surveillance
The regulations oblige manufacturers to carry out post-market surveillance, for example. To this end, manufacturers must draw up a post-market surveillance plan and collect and evaluate the post-market surveillance data in accordance with this plan. Manufacturers find it particularly difficult to analyze trends. The output then flows into reports such as the Periodic Safety Update Report (PSUR).
Depending on the class of the devices, the reports must also be published in EUDAMED.
b) Post-market clinical follow-up
One part of post-market surveillance is the post-market clinical follow-up (PMCF). This involves collecting clinical data to keep the clinical evaluation up to date. If the data is insufficient, PMCF studies may be necessary.
c) Vigilance
If serious incidents or trends leading to an unacceptable risk are observed in the post-market phase, manufacturers must notify the authorities (vigilance) and take corrective action.
d) Other
Post-market activities include monitoring new regulations (regulatory update), monitoring vulnerabilities in off-the-shelf software, and eliminating these vulnerabilities with security patches.
4. Persons responsible
These activities are not only the responsibility of manufacturers, for example, their product managers, quality management representatives, medical device consultants, and responsible persons.
Suppliers, importers, and distributors also have obligations in this post-production phase.
5. Interaction with risk management
The essential objective of all these activities is to ensure patient safety, i.e., to minimize risks from unsafe devices.
For this reason, the third edition of the risk management standard ISO 14971 has increased the requirements for the post-production phase. The associated Technical Report ISO/TR 24971 describes these requirements in more detail.
To assess the benefit-risk ratio, manufacturers must continuously monitor and consider the state of the art.
Not doing so is one of the most common mistakes in risk management.
6. Support
Do you still have questions about the post-market phase? Then, take advantage of our free micro-consulting.
If you want to delve deeper into the topic, we offer video training courses at the Medical Device University.
The post-market services take a large part of the work off your hands, from monitoring the regulations to post-market surveillance. This enables you to achieve:
- Regulatory safety and compliance
- Dependable, fast, and comprehensive monitoring and response
- Tracking of measures
- Cost reduction through automation
- Time for value-adding tasks (to compensate for the shortage of skilled workers)
Contact us if you would like support.
