The SSCP (Summary of Safety and Clinical Performance) is not the only report that the MDR requires from many medical device manufacturers. In terms of content, the SSCP even overlaps with the PSUR and the PMS reports.
The IVDR places almost identical requirements on the SSP (Summary of Safety and Performance).
How do all these reports differ from one another? How can manufacturers avoid redundant work and unnecessary expenses? This article provides answers and seven plus one tips.
1. SSCP/SSP: Why another report?
The SSCP (Summary of Safety and Clinical Performance) or SSP on the one hand and the PSUR (Periodic Safety Update Report) on the other both report on safety. However, they have different objectives, recipients, and requirements regarding when and how they must be created and updated (Tab. 1).
| SSCP, SSP | PSUR | |
| Recipients, target audience | Public (via EUDAMED), e.g., medical professionals, possibly patients | Internal, notified bodies, authorities |
| Objective | Informing, for example, to decide on the purchase or use of the device | Consolidate post-market surveillance data to decide on measures |
| Mandatory for | Implantable devices, class III medical devices, and class C and D IVDs | Medical devices in classes IIa, IIb, and III, as well as IVDs in classes C and D |
| Update | No requirements, typically in parallel with PSUR (at least annually) if new (clinical) data are available | For class IIa, at least every second year; for classes IIb and III, as well as for IVDs, at least annually |
| Language | Languages of the EU member states in which the device is to be sold. English is always included. | Typically English or the language of the manufacturer |
The SSCP, the Summary of Safety and Clinical Performance (or the SSP for IVD manufacturers), burdens manufacturers with an additional report. At the same time, it enables manufacturers to control and formulate information for the public in a more targeted manner.
2. What must the SSCP/SSP contain?
a) Content according to MDR and IVDR
When selecting the content, the manufacturers are subject to the legal requirements. These are defined in identical terms in the MDR in Article 32 and the IVDR in Article 29. Accordingly, the SSCPs must include
(a) the identification of the device and the manufacturer, including the Basic UDI-DI and, if already issued, the SRN;
(b) the intended purpose of the device and any indications, contraindications and target populations;
(c) a description of the device, including a reference to previous generation(s) or variants if such exist, and a description of the differences, as well as, where relevant, a description of any accessories, other devices and products, which are intended to be used in combination with the device;
(d) possible diagnostic or therapeutic alternatives;
(e) reference to any harmonised standards and CS applied;
(f) the summary of clinical evaluation as referred to in Annex XIV, and relevant information on post-market clinical follow-up;
(g) suggested profile and training for users;
(h) information on any residual risks and any undesirable effects, warnings and precautions.
b) MDCG requirements
In guideline MDCG 2019-9, the MDCG provides a chapter structure for the SSCP; analogously, MDCG 2022-9 provides a chapter structure for the SSP. The latter differentiates between products for self-testing (directed at patients/laypersons) and those not (directed at professional users and possibly patients/laypersons).
The chapter structure proposed by the MDCG specifies the contents even more precisely, which the MDR or IVDR requires. Fig. 1 shows a simplified example.
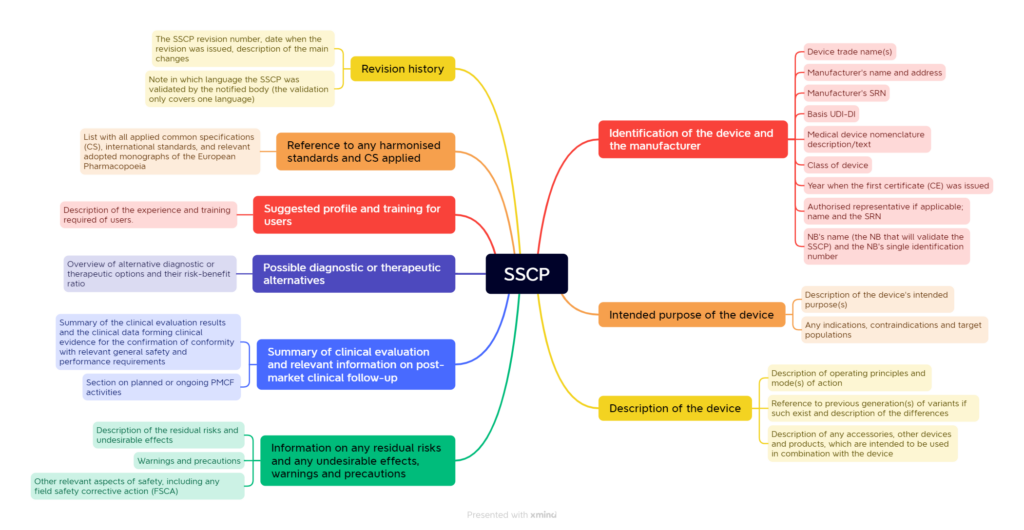
3. Where does the data come from?
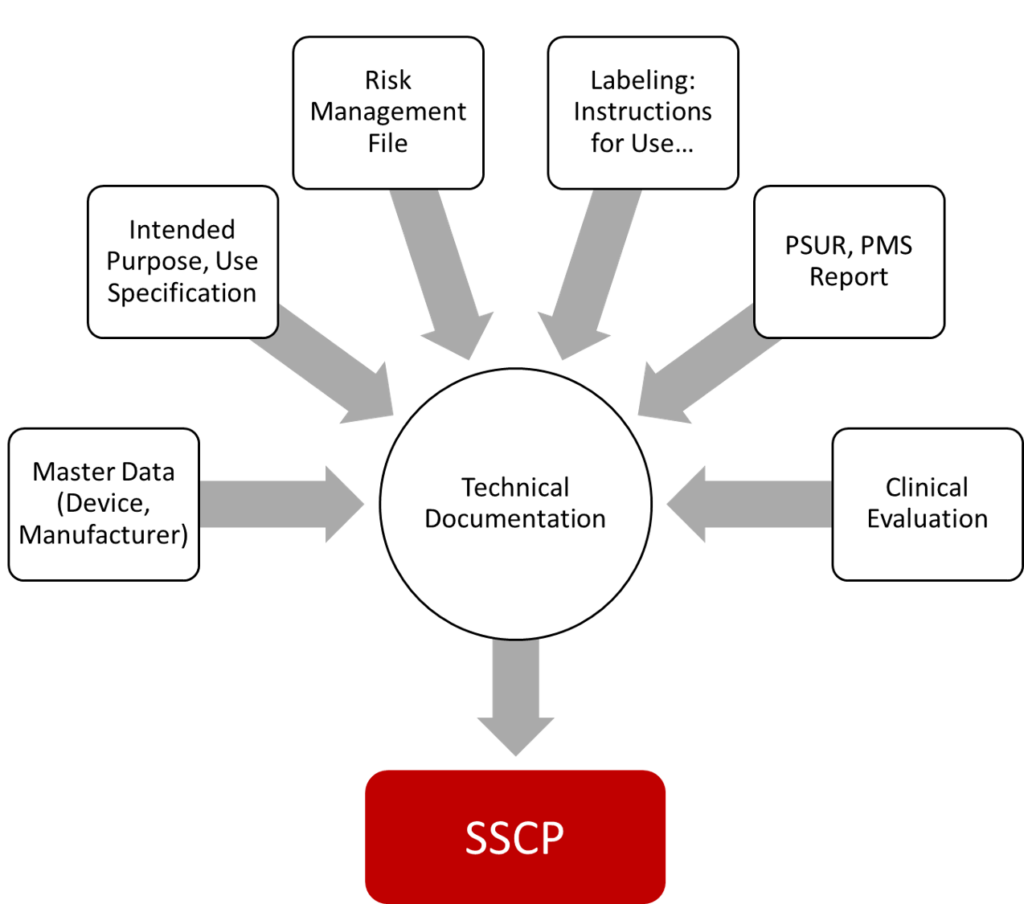
The content summarized in the Summary of Safety and Clinical Performance (SSCP) and the SSP comes almost exclusively from the technical documentation (see Fig. 2).
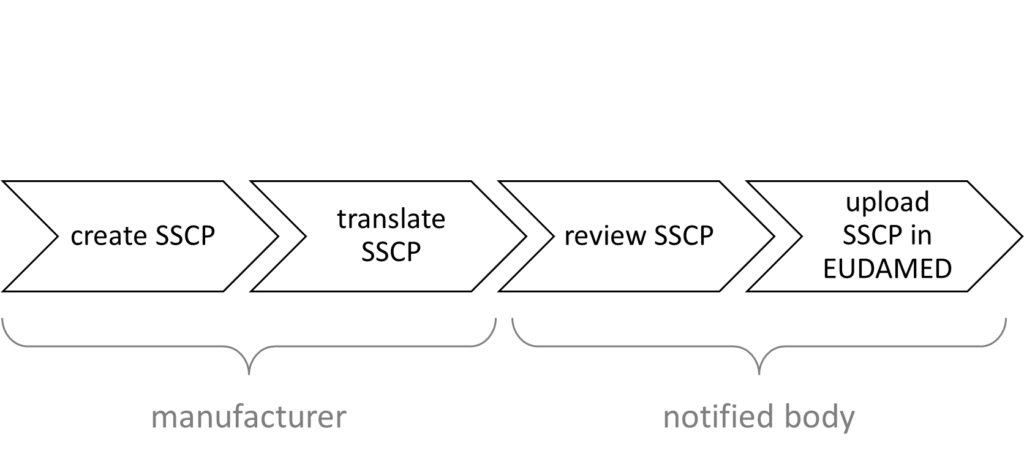
IVD and medical device manufacturers must translate the SSP and SSCP into the relevant languages of the EU target countries where the device will be marketed.
The manufacturers then send all versions to their respective notified body, which checks one (!) language version. The notified body checks whether the data in the summary report matches the data in the technical dossier. For example, it compares the information in the V&V reports, risk management, clinical evaluation or performance evaluation, post-market surveillance, PMCF/PMPF, and IFU with the information in the SSCP/SSP.
If successful, the notified body uploads all language versions to EUDAMED (see Fig. 3).
4. Tips for creating the SSCP
Tip 1: Involve the right people
The Summary of Safety and Clinical Performance can be understood as the essence of the technical documentation for the public.
Experts from the following areas should be involved in creating this summary report:
- clinical affairs (most important role)
- regulatory affairs (possibly legal)
- quality management, including the PRRC
- risk management
- product management
Tip 2: Update the report together with the PSUR
As soon as new data is available, the SSCP should be updated. This is the case (at the latest) when a new version of the PSUR (Periodic Safety Update Report) is available.
Tip 3: Avoid discrepancies between TD and SSCP/SSP
Informing the public – including competitors – about all risks and problems may be difficult and tempt you to “embellish” the data. But here, too, honesty really is the best policy.
You should also avoid unintentional discrepancies. Slightly different indications or differently formulated safety instructions can quickly lead to unnecessary discussions with notified bodies.
When reviewing the SSCP/SSP, it has proven beneficial to provide the notified body not only with the short report but also with an additional document that compares the contents of the SSCP/SSP with the corresponding TD documents, including chapters.
Tip 4: Use clear language
In contrast to clinical or performance evaluations, the SSCP or SSP should not be formulated like a scientific publication with many “insider abbreviations” and (incomprehensible) technical terms but should be tailored to the target group, which includes laypersons.
It may be helpful to divide the SSCP/SSP into areas for laypersons and specialist users. The MDCG 2022-9 even specifies this for IVDs.
Tip 5: Ensure a professional translation
DeepL and Google Translate provide great services. But they are no substitute for a professional translation. At the very least, the “fine-tuning” of the SSCPs/SSPs must be done by a person who has a perfect command of the target language and understands the content.
Regardless of which EU countries you want to market your product to, you will always need an English version of the SSCP/SSP.
Tip 6: Use the guidelines MDCG 2019-9 and 2022-9
You will save yourself discussions with your notified body if you adhere to both the chapter structure and the content specified by the MDCG 2019-9 and MDCG 2022-9 guidelines.
Tip 7: Don’t forget to integrate usability engineering
The summary report should be understandable to laypersons. This means that, in addition to the instructions for use, you have another document that you should consider in your review of usability engineering, e.g., in the summative evaluation.
Bonus tip: Automate the creation of the SSCP
Almost all of the information that must be included in the Summary of Safety and Clinical Performance comes from the technical documentation, which is often even identical in letters. Therefore, much of the work involves copying, structuring, and formatting content.
These are tasks that a computer can perform error-free in a fraction of a second.
5. Summary and conclusion
The Summary of Safety and Clinical Performance (SSCP) is mandatory for higher-class devices. For IVDs, this report is called the Summary of Safety and Performance (SSP). Both mean additional work for the device manufacturers.
These reports ensure transparency regarding the risks and effectiveness of the products. Not all manufacturers may like this, especially since the competition also gains insight.
But there is also good news:
- The obligation to publish an SSCP applies only to devices in higher risk classes.
- For safe and effective devices, the SSCP/SSP and EUDAMED create an additional channel through which manufacturers can communicate the quality of their products.
- Thanks to the guidelines MDCG 2019-9 and MDCG 2022-9, the report’s contents and structure are clear.
- No new data needs to be collected for the report. It is already in the technical documentation.
- Thanks to digitalization, the workload for creating the SSCPs and SSPs can be minimized.
The Johner Institute’s clinical affairs and IVD teams are always available to help with SSCP and SSP. They will answer your questions, train your colleagues, review your summary reports, and, if you wish, take over the creation of the SSCP or SSP for you.
Get in touch. Here you will find the contact details.



Thank you, Dr. Kuhnert, for this insightful article on the SSCP and its critical role in ensuring transparency and safety in medical devices. I appreciate the clear explanation of the regulatory framework and the step-by-step guide on preparing the SSCP document. Your detailed approach makes a complex topic much more accessible. This is invaluable for anyone navigating the medical device industry. Looking forward to more of your expert insights.
Best regards,
Jaseph