How to meet PMCF regulatory requirements as quickly as possible
Post-Market Clinical Follow-up is increasingly being criticized by the notified bodies. And this is after the manufacturers have successfully mastered the hurdle before: the initial clinical evaluation.
This article provides manufacturers of medical devices with
- a quick overview of the regulatory requirements for PMCF,
- a description of the key methods used in Post-Market Clinical Follow-up, and
- tips to avoid the most common non-conformities and, therefore, unnecessary delays and costs.
1. PMCF: The basics
a) Objective and definition
The MDR requires manufacturers to demonstrate the safety, performance, and benefits of their medical devices throughout the product lifecycle as part of the clinical evaluation. This evidence also requires clinical data. Consequently, manufacturers must collect and evaluate this clinical data throughout the complete product life cycle.
This continuous process of updating the clinical evaluation (and thus updating the clinical data) is called Post-Market Clinical Follow-up. This is also how it is defined in Part B of Annex XVI of the MDR:
“PMCF shall be understood to be a continuous process that updates the clinical evaluation referred to in Article 61 and Part A of this Annex and shall be addressed in the manufacturer’s post-market surveillance plan.”
MDR, Annex XIV, Part B
An assessment of whether the identified risks are still acceptable or whether new risks have been identified is part of the PMCF.
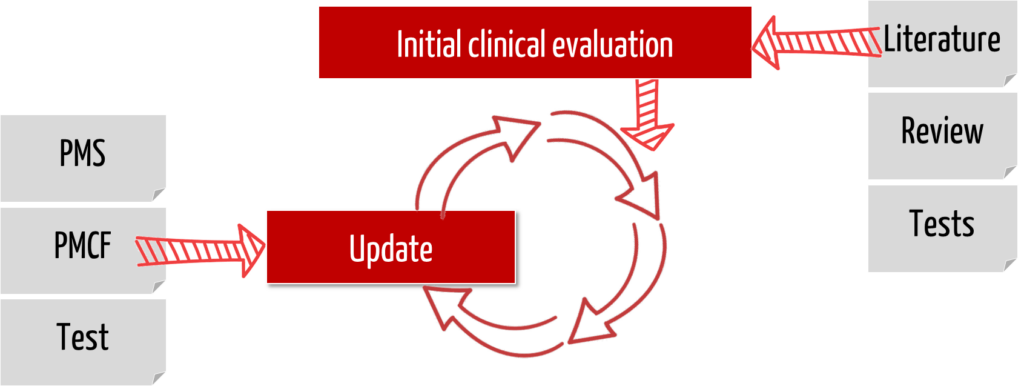
b) Distinction
The PMCF is a subset of Post-Market Surveillance (PMS). PMS involves collecting all types of information from the field, such as service reports, hotline calls, customer complaints, etc. The objectives of PMS are broader than the objectives of PMCF.
For a detailed distinction between PMS and PMCF, see this article on PMS.
2. Regulatory requirements
a) General requirements
Many jurisdictions require the continuous collection and evaluation of clinical data. However, the concept of Post-Market Clinical Follow-up comes from the MDR. This EU regulation requires in Article 10, Paragraph 3, that clinical evaluation include Post-Market Clinical Follow-up of medical devices.
In Annex II, Paragraph 6.1 (d) of the MDR, both the plan and the report of the Post-Market Clinical Follow-up (PMCF) are counted as clinical data. The documents are, therefore, part of the technical documentation of a medical device under the requirements of the MDR.
b) Requirements for the process
PMCF is conducted according to a methodology outlined in a PMCF plan. The MDR requires in Annex XIV, Part B, 6.1:
“The PMCF plan shall specify the methods and procedures for proactively collecting and evaluating clinical data with the aim of:
MDR, Annex XIV, Part B, 6.1
- (a) confirming the safety and performance of the device throughout its expected lifetime,
- (b) identifying previously unknown side-effects and monitoring the identified side-effects and contraindications,
- (c) identifying and analyzing emergent risks on the basis of factual evidence,
- (d) ensuring the continued acceptability of the benefit-risk ratio referred to in Sections 1 and 9 of Annex I, and
- (e) identifying possible systematic misuse or off-label use of the device, with a view to verifying that the intended purpose is correct.”
The notified body must verify that the PMCF plan and its implementation are adequate.
This review of the clinical evaluation by the notified body also includes the manufacturer’s procedures and documentation of PMCF measures, as well as justification for noncompliance with the PMCF, if applicable.
c) Requirements for the documentation
Documents overview
Notified bodies review in their Clinical Evaluation Assessment Report (CEAR) whether a PMCF plan is listed and PMCF activities are documented in a PMCF evaluation report:
“whether the post-market surveillance plan, including the PMCF plan, is adequate“
[…]
“Provide an assessment of the consistency of the clinical evidence with:
[…]
(b) the post-market clinical follow-up (PMCF) plan.”
MDCG-2020-13
To meet these objectives, the Medical Device Coordination Group (MDCG) provides medical device manufacturers with important guidance on PMCF documentation.
PMCF plan according to MDCG 2020-7
The template for a Post-market clinical follow-up (PMCF) Plan – a guide for manufacturers and notified bodies (MDCG 2020-07) comprises 12 pages that can be filled out by the manufacturer in a product-specific manner. The information is always requested in table format.
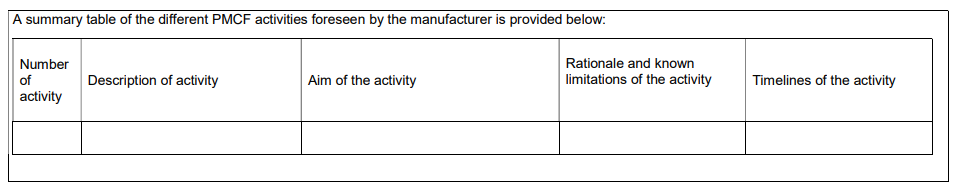
Section C lists the PMCF activities planned for the medical device. In doing so, manufacturers should first identify the objective of each PMCF activity and then provide a rationale for its appropriateness. It is also necessary to indicate deadlines within which the activities are to be performed. These deadlines should be quarterly or at least annually.
The template gives examples of PMCF activities that include the following:
- medical device registry – search
- PMCF study
- real-world evidence
- surveys
For each activity, add,
- from which document the claim of the planned PMCF activity comes,
- a description of the planned activity (general method and implementation),
- the objective of the PMCF activity,
- methodology that will be used as part of the activity,
- description of the rationale for the appropriateness of the chosen methodology, and
- deadline and timeline.
PMCF assessment report according to MDCG 2020-8
The PMCF assessment report, according to MDCG 2020-8, lists the outputs of the planned PMCF activities in accordance with the requirements of the MDR. It is a document of the technical documentation of your medical device.
The conclusions of the PMCF assessment report will be considered when updating:
- clinical evaluation
- risk management file
- PMCF plan
- Summary Safety and Clinical Performance report (SSCP).
Specifically, for class III devices and implantable devices, the PMCF assessment report will be updated at least annually based on this data. The PMCF assessment report is part of the clinical evaluation report and technical documentation.
d) Exceptions
Not all cases require post-market clinical follow-up activities. The MDR writes in this regard:
“(b) The post-market surveillance plan shall cover at least:
— a PMCF plan as referred to in Part B of Annex XIV, or a justification as to why a PMCF is not applicable.”
MDR, Annex III, 1.1. b)
A major problem is that there is no guidance on which product groups or classifications this can be applied to, nor examples of wording that can be used to provide justification.
Notified bodies review in their Clinical Evaluation Assessment Report (CEAR) whether a rationale is stated and adequate:
“If no PMCF is planned, has the manufacturer provided an acceptable justification for not conducting a PMCF?”
MDCG-2020-13
There are medical devices that do not have an intended medical purpose or for which the collection of clinical data is not useful. Examples include accessories and some medical devices, such as bedpan flushers for cleaning bedpans, or turbines for dental drills.
In these cases, where clinical data are inappropriate to demonstrate conformity with the general safety and performance requirements, a clinical evaluation is performed based on Article 61 (10). Then, the manufacturer may rely on other evidence, such as non-clinical test methods, including performance evaluation and technical inspection (“bench testing”). Since there are no clinically relevant endpoints, there is no need to collect PMCF data.
How can you determine if PMCF activities are appropriate, and to what extent? Approach it as you would a clinical evaluation for clinical evidence and consider the type, classification, intended purpose, and risks of the device.
This is also confirmed by MDR:
“That level of clinical evidence shall be appropriate in view of the characteristics of the device and its intended purpose.”
MDR, Article 61, 1
“The clinical evaluation shall be thorough and objective, and take into account both favourable and unfavourable data. Its depth and extent shall be proportionate and appropriate to the nature, classification, intended purpose and risks of the device in question, as well as to the manufacturer’s claims in respect of the device.”
MDR, Annex XIV, 2
To justify why PMCF activities are not necessary, use the formulations or considerations
- from MDCG 2020-6 to describe well-established technology,
- based on risk management and considering the specific characteristics of the interaction between the device and the human body, the intended clinical performance, and the device’s claims.
3. Types of PMCF activities
a) Overview and minimum requirement
The MDR distinguishes PMCF activities as general PMCF methods and procedures and specific PMCF methods and procedures:
(a) the general methods and procedures of the PMCF to be applied, such as gathering of clinical experience gained, feedback from users, screening of scientific literature and of other sources of clinical data;
(b) the specific methods and procedures of PMCF to be applied, such as evaluation of suitable registers or PMCF studies.
MDR, Annex XIV, Part B 6.2
As a minimum, product-specific literature searches must be conducted to obtain so-called pivotal data from reports published in peer-reviewed scientific literature.
The article on systematic literature research provides you with concrete assistance.
For implantable devices and class III devices for which clinical inspections have not been carried out in accordance with Article 61(4), the PMCF plan shall include post-market studies to confirm the safety and performance of a device.
b) Additional PMCF activities
Overview
Furthermore, the following activities are part of the Post-Market Clinical Follow-up:
| PMCF activity | description |
| post-market studies | PMCF studies are clinical investigations within the meaning of Article 74 MDR that are conducted after the device has been placed on the market. At this point, the device already bears a CE marking. |
| collection of data in registers | A manufacturer product register specific to the type of device or group of medical devices to which the device belongs. The qualitative and quantitative data – based on the risk of the device and associated accessory – to be collected and analyzed shall be defined in advance. |
| systematic questioning of patients/users – user questionnaires (survey) | Surveys planned to collect information on the use of the medical device in question |
| publications | Freely available documentation for your own device |
| real-world evidence analyses (RWE analyses) | Data accrues automatically and planning is dependent on objectives and recommended in terms of optimal use of data. There is no regulatory requirement here; no specific endpoints are tracked; there is no time limit and no case number planning. |
For more detailed guidance on PMCF studies, please refer to the corresponding article.
Clinical investigations
Clinical investigations are mostly confirmatory in nature and require elaborate study planning and a clear description of the study conditions. Each confounding factor can lead to an increased dispersion and thus have a considerable influence on the statistically necessary number of cases.
It is, therefore, not surprising that the study population should be kept as homogeneous as possible by means of suitable inclusion and exclusion criteria. This saves time and costs but also leads to ideal conditions under which the desired clinical endpoints of a medical device are reviewed. The extent to which the clinical data generated in this way still represents routine use may well be questioned in the case of one or the other study.
In contrast to clinical investigations, the use of medical devices can also be tested in everyday care. The study conditions and especially the selection of participants are not controlled by inclusion and exclusion criteria. The output of such pragmatic clinical investigations (PCT, pragmatic clinical trials) is real world data (RWD), which can be used to close the gaps between the experimental and the routine use of a medical device.
4. Common mistakes
a) Lack of product-specific clinical data
Equivalence consideration no longer possible under MDR
The previous strategy of clinical evaluations under the MDD largely involved evidence via clinical data on equivalence products. The requirements for demonstrating equivalence have increased under the MDR.
However, in the post-approval process, the collection of further clinical data on the actual device was neglected. This leads to the fact that
- long-term effects of devices were not collected and evaluated,
- not considered risks remained undiscovered, as well as
- extensions of the clinical indications were not taken into account.
Requirement of PMCF studies for MDR certification
The explicit requirement for proactive collection of clinical data in the PMCF process is intended to address these issues. They are used to improve the safety of medical devices and to continuously review their predicted performance.
In general, there must be sufficient clinical evidence to confirm the safety, performance, and acceptability of the benefit-risk determination in relation to the state of the art for legacy devices prior to CE marking under the MDR. PMCF studies may even need to be conducted to address the defect in clinical data.
MDCG 2020-6 provides for PMCF studies in the absence of equivalence and for product-specific risks:
“The European Commission guidance MEDDEV 2.12/2 regarding PMCF studies notes different instances where a PMCF study may have been justified:
– Route chosen for clinical evaluation: where CE marking for legacy devices was based upon equivalence, PMCF studies may have been necessary. The European Commission guidance MEDDEV 2.12/2 regarding PMCF also notes that in the case that clinical evaluation was based exclusively on clinical data from equivalent devices for initial conformity assessment, the certifying notified body shall verify that PMCF studies have been conducted, in accordance with the relevant provisions of the Directives.
– Device related factors: There are a number of device-related factors where PMCF studies may have been necessary.
When assessing the conformity of legacy devices under the MDR, it is important to verify whether PMCF studies considered necessary under the MDD/AIMDD (and where applicable, during the transition period, under the MDR), have been appropriately conducted, and results are taken fully into account for in the clinical evaluation for the conformity assessment under MDR.“
MDCG 2020-6
Conclusion: such evidence should not rely on new PMCF studies initiated under MDR to fill gaps (e.g., indications not supported by clinical evidence).
b) No consideration of off-label data
Off-label use for indication expansion
Manufacturers often question whether off-label data can be used to expand the intended purpose or indications. How to handle these data is described in the Team-NB position paper.
“Off-label use” of a medical device is generally understood to occur when a device is used outside of its approved use, i.e., not as described in the instructions for use:
- outside of specific patient populations, e.g., in pediatrics
- for a different stage or severity of the disease
- for a similar (not identical) clinical condition
- introduction into the body via other routes
The term “off-label use” is mentioned in the MDR in Annex XIV, Part B in the context that the manufacturer’s PMCF plan must identify systematic misuse or off-label use of the device to review whether the intended purpose of the device is correct. However, “off-label use” or “misuse” are not defined in the MDR.
Off-label data may be quantitatively sufficient, especially when systematic off-label use has been identified, but they often lack sufficient quality in terms of meaningful conclusions. Off-label data are typically collected outside of formal protocols, and the lack of protocols ultimately results in insufficient quality and an inability to draw evidence-based conclusions.
“Foreseeable misuse”, “unsystematic misuse”, and “systematic misuse”
Manufacturers must identify foreseeable misuse through usability studies, or clinical investigation reports prior to placing on the market. However, it is often difficult to predict areas of future misuse, and without the manufacturer’s direct oversight of each device’s use, it is inevitable that misuse will occur. When misuse is identified, the manufacturer must eliminate or control the risks in accordance with risk control measures.
Please also note the article on usability studies and the article on risk management.
The manufacturer should differentiate between systematic misuse and unsystematic misuse in documented off-label use:
| unsystematic misuse | systematic misuse | |
| details | In cases of unmet medical need (“unmet medical need”) and when no other approved viable alternatives are available, it might be ethical for a physician to consider alternative options. A device was used “unsystematically,” i.e., incidentally. | A device is repeatedly or continuously used outside of its approved intended purpose and indications. |
| sources | Such use of a device is often reported in the literature in the form of single case studies. Manufacturers may identify such off-label use as part of their general post-marketing activities through literature searches. | Literature, user survey |
| clinical data | yes | yes |
| consideration in clinical evaluation | yes | yes |
| documentation in the PMCF report | no | yes |
| extension of the intended purpose | no | no |
When manufacturers identify systematic off-label use, they should take appropriate action not only to curb misuse but also to determine whether there is a genuine need in the medical community for the newly identified use in relation to the specific medical purpose/indication.
c) Periods of documentation and implementation of the measures are not appropriate
The MDR specifies timeframes for the preparation of the PSUR, which must include the key outputs of the PMCF process. However, this does not mean that the performance and evaluation of PMCF activities are linked to it.
There are activities that should be conducted more frequently, such as a literature review. Furthermore, interim analyses of PMCF studies or user surveys provide information to derive appropriate measures or to make adjustments to the study protocol or design, e.g., in case of changes in the number of patients or study centers.
The MDR differentiates in the documentation of PMCF activities with regard to the classification of devices:
For class III devices and implantable devices, the PMCF evaluation report and, if indicated, the summary of safety and clinical performance referred to in Article 32 shall be updated at least annually with such data.
MDR, Article 61, 11
In each case, a summary of the main outputs of the PMCF assessment report is required in the PSUR. This means that the PMCF assessment report contains a detailed analysis and evaluation of the PMCF data (MDCG document 2020-8), and “only” the main points are included in the PSUR (MDCG document 2022-21).
Now, during a two-year period for PSUR preparation, multiple PMCF assessment reports may be prepared because the interval for doing so is shorter or there are multiple activities with different intervals. It is recommended that a brief summary of each PMCF assessment report be included in the PSUR.
In any case, before each PSUR is prepared, it is appropriate to go through all the PMCF analyses and assessment reports that have been incurred during the period being assessed and see if everything has been completed. If PMCF activities have not yet been completed, the PSUR must explain why there may have been a deviation from the plan or why sufficient PMCF outputs are not yet available.
For further references, see the article on the PMS report or PSUR.
5. Conclusion and support
Medical devices should be safe, efficient, and effective throughout their life cycle. PMCF activities are a measure required by the MDR and the essential method to demonstrate this. However, this does not mean that PMCF processes or PMCF studies are always mandatory.
The scope should be appropriate to the nature, classification, intended purpose, and risks of the device in question.
As part of the clinical evaluation, gaps in clinical data are identified, and initial proactive PMCF activities are defined.
Get the appropriate support in the preparation of your entire clinical evaluation file as well as in the design, implementation, and evaluation of your PMCF activities. The Johner Institute offers:
- outsourcing your clinical evaluation and planning your clinical data collection
- clinical evaluation seminars to acquire the necessary competencies
Contact us right away with a proposed appointment for a free, non-binding meeting.
Contact the experts at the Johner Institute. They will review your clinical evaluation file and PMCF documents and give you concrete advice for improvement. They will also support you with case number planning and analysis of your PMCF data.


