Orphan medical devices are medical devices (and IVDs) for small patient groups. The development of these niche products is often not profitable for manufacturers. As a result, particularly vulnerable groups, such as children, do not always receive adequate medical care.
This article shows possible solutions and classifies the guideline MDCG 2024-10.
1. Orphan medical device: Definition and examples
1.1 General definition
The term orphan medical device (sometimes also orphan device) was transferred from the “orphan drugs” to medical devices.
The term “orphan drugs” refers to drugs for treating rare diseases. Depending on the legislation, the criteria for this classification range from one to eight patients per 10,000 inhabitants. Accordingly, orphan medical devices can be defined.
A medical device or IVD whose intended purpose is specifically aimed at small patient groups, where “small” refers to no more than (for example) 0.5 per mille of the population
Intended purposes that target these small patient groups are also referred to as orphan indications, and the patient groups as orphan populations.
1.2 MDCG definition
In the guideline MDCG 2024-10, the Medical Device Coordination Group (MDCG) uses its own definition.
the device is specifically intended to benefit patients in the treatment, diagnosis, or prevention of a disease or condition that presents in not more than 12,000 individuals in the European Union per year; and at least one of the following criteria are met:
- there is insufficiency of available alternative options for the treatment, diagnosis, or prevention of this disease/condition, or
- the device will offer an option that will provide an expected clinical benefit compared to available alternatives or state of the art for the treatment, diagnosis, or prevention of this disease/condition, taking into account both device and patient population-specific factors.
For the MDCG, a medical device only counts as an orphan device if it is intended not only for a very small patient group but also if its absence would worsen the care of this orphan population.
1.3 Definition of limit values
The MDCG sets the limit at 12,000 patients per year in the EU. This corresponds to (with a population of 450 million) about 0.27 per thousand.
This value is low compared to other definitions:
- In the USA, orphan drugs affect a maximum of 0.75 per thousand of the population (almost three times as many).
- The EU sets the value at five in 10,000 persons, i.e., 0.5 per thousand (almost double) in the Regulation “on orphan medicinal products.”
It is not generally known what prompted the MDCG to set this value so low.
Whether the number of patients is so low because the disease is so rare or because the population is so small (e.g., only premature babies) is irrelevant for the definition.
1.4 Examples
An example of an orphan device is a heart valve implant intended to treat a rare subpopulation of patients with valvular heart disease characterized by specific anatomical features, such as extreme dilation of the ventricular outflow tract.
Other examples include devices intended to treat a rare subpopulation of an otherwise non-rare condition requiring definitive intervention in the neonatal period (e.g., a rare subpopulation of hemodynamically significant patent ductus arteriosus requiring acute surgical closure).
2. Problem
Developing medical devices is expensive. It is particularly difficult to recoup this investment for orphan devices for several reasons.
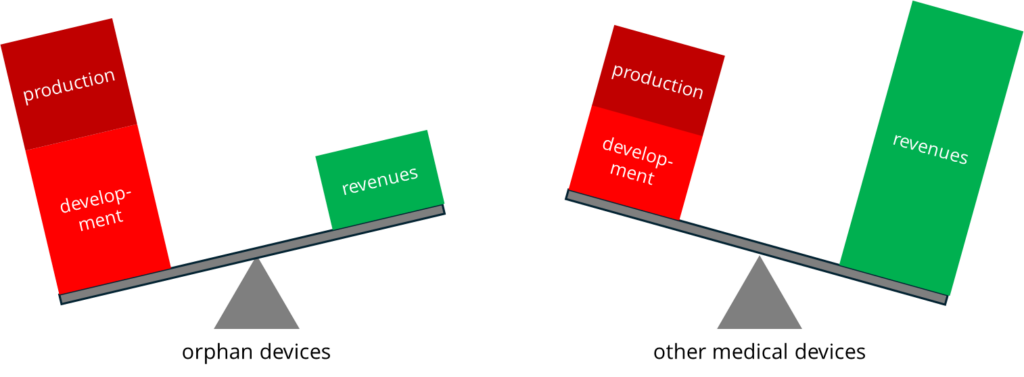
2.1 Very high costs for the development of orphan devices
The costs for the development of orphan medical devices are particularly high for several reasons:
- The development is very demanding. For example, small versions of devices that are then intended for children cannot be realized with the same materials or material thicknesses.
- Rare diseases are often more difficult to diagnose and treat. The research costs are correspondingly high, as are the development risks.
- Clinical evaluation is expensive because it takes a particularly long time to reach the number of cases required for evidence. Recruiting patients is more time-consuming and costly than for a device for large patient groups.
2.2 Very high product (ion) unit cost
Devices for a small number of patients are only manufactured in small quantities. As a result, manufacturers do not benefit from the economies of scale in a mass market.
2.3 Low revenue
Devices for rare diseases are rarely needed. The expected revenues are correspondingly low.
2.4 Interim conclusion
Low revenues offset the high costs of development and production. Manufacturers usually cannot compensate for this balancing act by increasing selling costs. As a result, they no longer offer devices for orphan populations. Inadequate medical care can be the consequence.
This under-supply affects these patients in two ways:
- Manufacturers do not develop medical devices and IVDs specifically for this patient group.
- Manufacturers exclude orphan indications from the intended purpose for broader patient groups because the costs for the specific clinical evidence would be too high.
3. Solutions
3.1 Regulatory solutions
The “regulatory compliant” way would be special approval procedures for these patient groups. This could be based on the FDA’s Humanitarian Device Exemption (HDE). This is based on the Orphan Drug Act and allows simplifications in the approval of products for rare diseases.
Alternatively, agreements and guidelines are conceivable that
- explicitly or implicitly weaken the legal requirements,
- allow exceptions (e.g., for devices that have already been placed on the market in the same or other markets) or
- exempt deviations from certain legal requirements from (criminal) prosecution.
The MDCG’s approach is described in subsection 3.3 of this article.
3.2 Funding
Developing and marketing medical devices and IVDs should also make economic sense for their manufacturers. The legislator has the opportunity to intervene in this market not only in a regulatory way but also by providing support:
- Subsidizing the devices or their use via health insurance companies or special tax breaks
- Financing the promising development of products for rare diseases via tenders
- Supporting research at universities and hospitals in order to partially save manufacturers the costs of research and basic development
3.3 MDCG 2024-10
The EU (now) recognizes that the MDR’s significantly increased requirements, particularly regarding clinical evaluation, represent a major challenge for the manufacturers of niche products.
3.3.1 Scope
The guideline MDCG 2024-10 is aimed at manufacturers of niche products, as well as the responsible authorities and notified bodies. The guideline concerns the MDR, not the IVDR.
In addition, the devices must meet the definition presented in section 1.2 to be considered orphan devices. Manufacturers must scientifically demonstrate compliance with these requirements.
3.3.2 Solutions
The MDCG would like to provide guidance to stakeholders on clinical evaluation.
- It points out permitted restrictions in the clinical data collected prior to placing the device on the market.
- In particular, it considers extrapolating data from other populations acceptable under certain circumstances, such as if they can be compensated for by post-market activities (PMCF).
- It also draws attention to other data sources, such as predicate devices, registries, or off-label use.
Secondly, it aims to support the “approval” process by
- providing notified bodies with guidance on assessment (e.g., “certificates with conditions”) and
- describing the role of expert panels. However, it remains unclear how manufacturers benefit from this.
3.3.3 Evaluation of the guideline
First, the positive news: It has been recognized that action is needed to avoid further hindering the medical care of orphan populations.
The authors of the guideline MDCG 2024-10 were faced with the seemingly impossible task of simplifying the development and approval of orphan devices, which has been hampered by the MDR, without undermining the rules of the MDR. They are not authorized to do so.
The result consists primarily of guidance such as explanations, and argumentation aids on how to use the leeway that the MDR already provides – but which is not always used by notified bodies, for example.
The added value of the guideline is that it can be referred to if you want to avoid an overly strict interpretation of the MDR.
The fact that the MDCG 2024-10 itself remains vague in many crucial places or describes matters of course limits the benefit. Editing might have increased the quality.
4. Summary and conclusion
4.1 Without orphan medical devices, there is a problem
It is not particularly attractive for manufacturers to develop medical devices for very small patient groups. This is especially true when laws such as the MDR increase the expenses further.
As a consequence, the EU is thwarting its own objectives for these patient groups, namely, to contribute to healthcare with safe, high-performance, and effective devices.
4.2 This can hardly be solved by guidelines
Guidelines such as MDCG 2024-10 can only compensate for this to a limited extent because they must not contradict legal requirements. Therefore, statements that the guideline would allow restrictions on clinical data for orphan devices are at least misleading.
Rather, the guideline points out the existing (!) degrees of freedom. No more and no less. This does not eliminate the problem.
4.3 Therefore, other approaches are necessary
If we want “minorities” such as “orphan populations” to have access to equivalent health care, we have to do more:
- Write laws that are designed to explicitly achieve these objectives
- Return to a risk-based approach
(Risks are not minimized by taking medical devices off the market because then the risk of “non-care” arises.) - Provide funding to ensure the care of these and future patient groups with rare diseases (this also requires investment in innovation for future or previously undiscovered diseases)
4.3 Conclusion
Dealing with particularly vulnerable groups such as children or patients with rare diseases highlights the problem that laws can have negative effects on these groups.
Attempts are being made to mitigate these effects by means of guidelines or new laws, such as the reporting requirement for devices that are no longer available. However, there is a risk that these attempts will be ineffective or have secondary consequences.
Short- and long-term measures can help:
| short-term measures | long-term measures |
| set up a funding program for orphan medical devices in the MDR/IVDR, allow risk-based exemptions, e.g., for legacy devices or devices that have been “approved” in other countries | establish proactive monitoring of gaps in care define and prioritize the objectives of the legislation establish regulatory science, e.g., to model systems amend laws (e.g., add new approval procedures to the MDR) based on these findings |
The Johner Institute already supports legislators and authorities outside the EU in these activities.


