Medical writers have a firm place in the ecosystem of medical device and IVD manufacturers. This article clarifies
- what regulatory requirements medical writers must fulfill,
- what these persons do, and
- what characterizes good medical writers.
1. Summary
A medical writer is a person who collects and evaluates research results and information on the use of products and medical information in the field of pharmaceutical and medical device research and documents them in an understandable, scientifically correct, and legally compliant manner.
Medical writing refers to creating scientific and technical documents in the regulated medical, medical device, and pharmaceutical fields.
2. The tasks of a medical writer
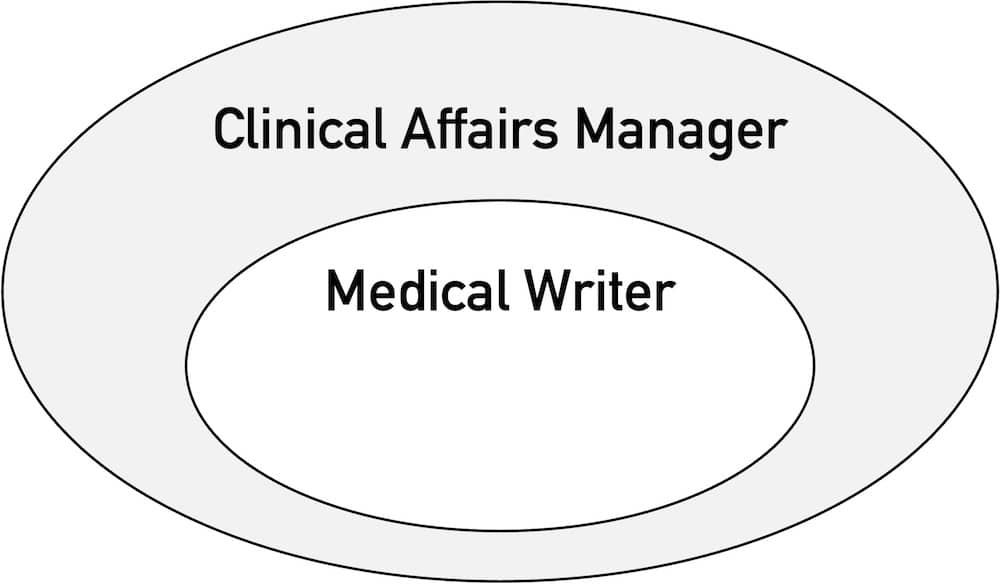
In the field of medical devices, medical writing is one of the tasks of clinical affairs managers. It includes the following activities:
- Systematic literature research (focusing on the state of the art and the device to be evaluated)
- Systematic evaluation and analysis of the data/findings obtained
- Creation of documents for the technical documentation (focus: clinical evaluation)
- Writing medical texts that must take regulatory requirements into account, e.g., patient information and training documents
- Writing scientific articles
The range of tasks performed by clinical affairs managers extends beyond medical writing. It also includes:
- Defining clinical strategies
- Evaluating preclinical and clinical data
- Specification of parameters and acceptance criteria for demonstrating performance
3. The regulatory framework
Laws such as the MDR and IVDR and standards such as ISO 13485 require a manufacturer to specify and ensure that its employees have the necessary competencies. However, they do not place any specific requirements on the role of medical writer.
Requirements for medical writers can be found in guidelines such as MEDDEV 2.7/1 rev. 4 and the planned MDCG guideline. These require the following as prerequisites for authors of clinical evaluations:
- a degree from higher education in the respective field and 5 years of documented professional experience; or
- 10 years of documented professional experience if a degree is not a prerequisite for a given task.
Elsewhere, the wording is even more specific:
e.g., post-graduate experience in a relevant science or in medicine; training and experience in medical writing, systematic review, and clinical data appraisal
Regardless of the competence requirements, the laws place requirements on the clinical evaluation plans and reports, i.e., on the work results of the medical writers.
4. Characteristics of good medical writers
The competence of a medical writer is reflected in the quality of the work results and the speed with which they are produced.
The hallmarks of good work results are:
- The texts are technically precise and correct and meet regulatory requirements.
- The analysis and evaluation of the (clinical) data is comprehensible and understandable and demonstrates a systematic, thorough, and objective approach.
- The critical evaluation considers the relevant scientific literature on safety, performance, and design characteristics (MDR, Art. 61(3)).
When discussing the state of the art with our customers, it usually quickly becomes clear whether someone is “only” able to write in a scientifically sound manner (usually with good results) or whether they are also familiar with the regulatory requirements and take them into account.
A good writer describes the “state of the art” in the clinical evaluation and formulates it in accordance with the requirements of the MDCG guidelines (MDCG 2020-5 and 2020-13).
Experienced medical writers can write clinical evaluation reports for many devices quickly by skillfully grouping the devices and reusing data where possible.
Professional medical writers avoid these common mistakes:
- The research is not understandable.
- The systematics of the research are not recognizable (e.g., no PICO scheme was used).
- The regulatory focus of the research is not recognizable (e.g., is it about the state of the art specific to the device under assessment?).
- The evaluation criteria have not been defined or applied.
- The evidence for the results is not sufficiently justified.
5. Conclusion
Clinical evaluations are the focus of the authorities and notified bodies. This is not surprising because the clinical evaluations summarize and confirm all statements regarding the safety, performance, and effectiveness of medical devices and IVD – and, thus, the conformity of the devices.
Therefore, manufacturers should ensure the competence of their medical writers, for example, through qualifications, in order to bring knowledge into the company and avoid regulatory trouble.
The Johner Institute trains medical writers. It also offers seminars on clinical evaluation and e-learning programs, among other things.
Please get in touch with us if you would like to find out more.


