The ISO 15189 standard “Medical laboratories – Requirements for quality and competence” specifies the requirements for a quality management system for medical laboratories. Laboratories are legally obliged to establish a QM system. According to ISO 15189, laboratories that operate in-house IVD medical devices require a QM system, which must be extended by additional chapters.
This article
- describes the legal basis,
- provides a quick overview of ISO 15189, and
- offers practical tips to help you expand existing QM systems as quickly and easily as possible, thereby meeting the regulatory requirements of the IVDR.
1. ISO 15189:2022: The basics
a) Scope of ISO 15189:2022
ISO 15189, “Medical laboratories – Requirements for quality and competence,” is primarily intended for use in medical laboratories. However, it can also be used in other health institutions, such as blood banks and transfusion services, and in a scientific context.
With the current version, the standard can also be applied in the context of near-patient testing.
The contents regulate how the QM system is created and operated in medical laboratories and how the competence of laboratory personnel is assessed and ensured. To this end, the standard formulates requirements for the organization, personnel, equipment, processes, and quality management.
b) Objectives of the standard
The standard’s objective is to improve the quality of medical laboratories and, thus, patient safety while strengthening trust in their work.
The key aspects are ensuring dependable test results, protecting patient data, and continuously improving laboratory services.
c) Specifics and benefits of ISO 15189
In contrast to standards such as ISO 9001 or ISO 17025, ISO 15189 is specifically tailored to the requirements of medical laboratories.
The standard confirms specific competence in medical tests and considers ethical aspects and patient safety.
In contrast to ISO 17025, which applies to general testing and calibration laboratories, ISO 15189 focuses on the specific needs of medical laboratories, including pre- and post-analytical processes.
Implementing ISO 15189 often optimizes laboratory processes, saves resources, and minimizes the risk of errors. This strengthens the confidence of patients, medical personnel, and authorities in laboratory services and can be a significant competitive advantage.
d) Structure of the current version
ISO 15189 is not a pure quality management system standard, even though it is often referred to as such. Its content goes well beyond that of a classic QMS. The actual QM system is described in Section 8, “Management system requirements.”
Section 3, “Terms and definitions” of ISO 15189, only refers to management systems and no longer to quality management systems. It is added that both terms are to be used synonymously.
In Section 3.17, the standard defines a management system as “a set of interrelated or interacting elements of an organization to establish policies and objectives, and processes to achieve those objectives.”
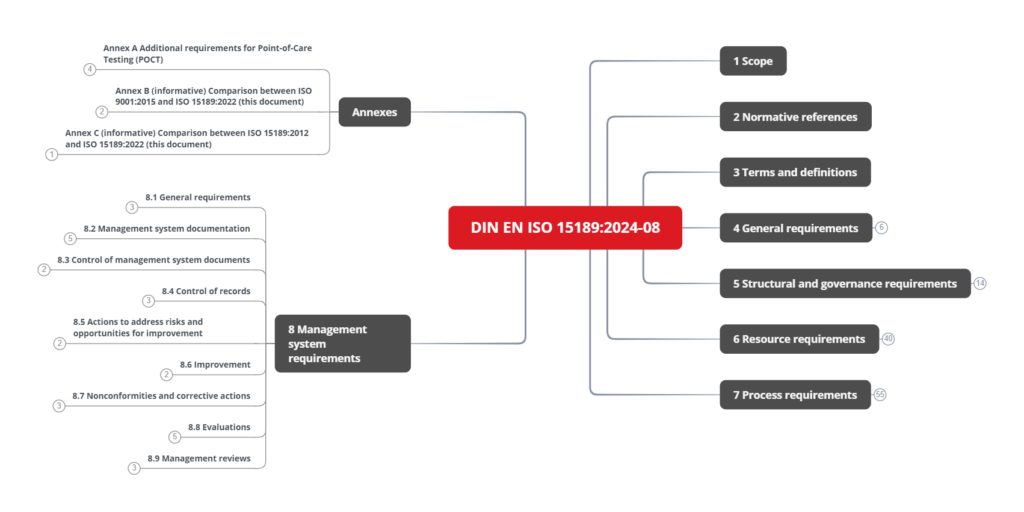
The normative requirements are divided into five sections and Annex A:
- Section 4: General requirements
- Section 5: Structural and governance requirements
- Section 6: Resource requirements
- Section 7: Process requirements
- Section 8: Management system requirements
- Annex A (normative): Additional requirements for Point-of-Care Testing (POCT)
e) What’s new in ISO 15189:2022 compared to its predecessor
ISO 15189:2022 is the standard’s fourth and currently valid main version (the German sub-version valid at the time of writing is DIN EN ISO 15189:2024-08), following the versions from 2003, 2007, and 2012. The changes to the previous version were moderate and mainly related to the standard’s chapter structure.
Contact us (e.g., via our contact page) so we can send you the version mapping free of charge. It will make the gap analysis easier for you.
Further adjustments relate to the significantly greater focus on risk management. For example, the standard repeatedly references ISO 22367 “Medical laboratories – Application of risk management to medical laboratories.”
f) Replacement of ISO 22870:2016
ISO 15189:2022 also replaces the standard ISO 22870:2016, which has been withdrawn without replacement. Content from ISO 22870 can be found in Annex A, “Additional requirements for Point-of-Care Testing (POCT).”
2. Legal requirements for QM systems in medical laboratories
Several laws and regulations set requirements for medical laboratories. These overlap, but in some cases, they have a different focus depending on the laboratory’s role and functions.
a) Requirement for all diagnostic laboratories in Germany
The Medical Devices Operator Ordinance (MPBetreibV) requires laboratories to comply with the Guideline of the German Medical Association for Quality Assurance in Laboratory Medical Examinations (Rili-BÄK) as a minimum requirement for a QMS.
Requirements of the MPBetreibV for the QMS:
Anyone who carries out laboratory medical examinations must set up a quality assurance system in accordance with the state of the art in medical science and technology to maintain the necessary quality, safety and performance when using in vitro diagnostic medical devices and to ensure the reliability of the results obtained before commencing this activity. Proper quality assurance in accordance with sentence 1 is assumed if the guideline of the German Medical Association on quality assurance of laboratory medical examinations in the version of May 30, 2023 (Deutsches Ärzteblatt of May 30, 2023, DOI: 10.3238/arztebl.2023.rili_baek_QS_Labor) is observed.
Translated from MPBetreibV, § 10 Section 1
b) Requirements for hospitals in Germany
The German Social Security Code, Book V (SGB V), contains requirements for a QMS that are aimed at physicians, care centers, and hospitals, among others. The Joint Federal Committee specifies these.
Requirements of the SGB V for the QMS:
Contracting physicians, medical care centers, licensed hospitals, providers of preventive services […] are obliged, in accordance with §§ 136 to 136b and 137d, to
1. participate in cross-institutional quality assurance measures that have the particular objective of improving the quality of results and
2. introduce and further develop quality management within the institution, which in hospitals also includes the obligation to implement patient-oriented complaint management.
Translated from SGB V, § 135a Section 2
c) Requirements for laboratories with in-house IVD medical devices
IVDR requirements
The regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) introduced uniform rules for the operation of in-house IVD medical devices throughout Europe in 2017. These also include strict requirements for the QMS, which must be met after the end of the transition period since May 26, 2024.
IVDR requirements for the QMS:
b) manufacture and use of the devices occur under appropriate quality management systems;
c) the laboratory of the health institution is compliant with standard EN ISO 15189 or where applicable national provisions, including national provisions regarding accreditation;
IVDR, Article 5 Section 5
Conclusions can also be drawn from the further content of IVDR, Article 5(5), regarding the required QMS.
Article 5(5) of the IVDR requires a QMS according to ISO 15189 but no accreditation according to this standard. Although accreditation has many advantages, the focus should first be on compliance with the standard’s, the IVDR’s, and national legislation’s requirements. Accreditation can be obtained at a later date if required.
MDCG requirements
The document “Guidance on the health institution exemption under Article 5(5) of Regulation (EU) 2017/745 and Regulation (EU) 2017/746” from the Medical Device Coordination Group MDCG 2023-1 interprets Article 5(5) of the IVDR. It provides a lot of text on the subject of QMS but can be summarized in a few words: The QMS should be designed to cover the requirements of Article 5(5) and Annex I of the IVDR.
In particular, one sentence of MDCG 2023-1 clarifies why there are two separate QMS requirements in Article 5(5), points b) and c).
However, as the manufacturing process of a device and the compliance to the relevant requirements of Annex I is not in the scope of this standard, compliance with EN ISO 15189 alone does not constitute an appropriate QMS for the manufacture of in-house IVDs.
MDCG 2023-1, 3.5.2
And the authors are right about that. ISO 15189 (as well as the Rili-BÄK) does not include a chapter on developing in-house IVD medical devices. However, the classic outputs of a corresponding part of the QMS are required in Article 5(5) and Annex I of the IVDR. Accordingly, the ISO 15189 system must be extended.
The MDCG recommends using harmonized ISO standards wherever possible to implement a suitable QMS. The only standard currently in question is ISO 13485, “Medical devices – Quality management systems – Requirements for regulatory purposes.”
Conclusions
Accordingly, it can be concluded from the contents of the IVDR and the MDCG that a QMS for diagnostic laboratories should be structured in the following way:
- Complete QMS according to ISO 15189 (or Rili-BÄK)
- Procedures for the development, manufacture, and change of in-house IVD medical devices (e.g., according to ISO 13485, Chapter 7)
- Procedures for surveillance of devices
- Procedures for incident reporting
- Procedures for obtaining information about equivalent CE-marked devices available on the market (equivalence analysis)
The risk management standard ISO 22367, mentioned several times in ISO 15189, also requires that ISO 13485, 7.3, be considered in the development of in-house IVD medical devices.
In this article, you can learn more about the IVDR requirements for laboratories with in-house IVD.
The new ISO 5649 standard “Medical laboratories – Concepts and specifications for the design, development, implementation and use of laboratory-developed tests” describes a possible development process for in-house IVD.
3. Quality management standards for laboratories
Some medical laboratories have implemented different QM systems. The decision to favor a system depends, among other things, on national requirements, the field of activity, and the age of the health institution.
a) Rili-BÄK Part A
The first part of the Guideline of the German Medical Association on Quality Assurance in Medical Laboratory Examinations (Rili-BÄK) “sets out the basic requirements for quality management and quality assurance for medical laboratory examinations in the field of medicine ” (Rili-BÄK Part A 1). In Germany, compliance with the Rili-BÄK is a legal requirement for diagnostic laboratories and is subject to review by state authorities.
b) ISO 15189
The standard ISO 15189 “Medical laboratories – Requirements for quality and competence” specifies, as the title suggests, the requirements for quality and competence in medical laboratories. If all requirements are met, laboratories can assume that they are also complying with the requirements of the Rili-BÄK because the content of both documents is very similar. However, ISO 15189 is more precise in formulating the requirements.
c) ISO 9001
Some laboratories have set up and certified a QMS according to ISO 9001 “Quality management systems – Requirements.” This “mother of QM systems” is very general and not laboratory-specific. Compared to ISO 15189, such a system does not cover all the requirements of Rili-BÄK.
d) ISO/IEC 17025
Some laboratories are also accredited according to ISO 17025, “General requirements for the competence of testing and calibration laboratories.” This standard has existed since 1999, and its direct predecessor, ISO Guide 25, is much older than ISO 15189. That is probably why so many laboratories are still accredited according to ISO 17025. However, this is changing; laboratories are switching to ISO 15189, following the recommendation of European Accreditation.
e) ISO/IEC 17020
ISO 17020, “Conformity assessment – Requirements for the operation of various types of bodies performing inspection,” is currently still the standard in pathology. Just as with laboratories accredited according to ISO 17025, it can be assumed that more and more laboratories will switch to ISO 15189 over the next few years.
4. Practical tips
Tip 1: Proceed in five steps
Step 1: For systems that deviate from ISO 15189 (ISO 17025, ISO 9001, etc.):
- Gap analysis to ISO 15189:2022
- Close gaps
- Eliminate unnecessary processes
Step 2: Create SOPs for surveillance of laboratory analyses and for incident reporting
Step 3: Adapt risk management to the requirements of ISO 22367
Step 4: Introduce a suitable development process in accordance with ISO 13485, Section 7
Step 5: If necessary, accreditation
Tip 2: Focus on one standard
Focus on ISO 15189 instead of integrated systems from, for example, ISO 9001 and ISO 17025. Certification according to ISO 9001 is unnecessary and has little significance.
Tip 3: Involve the (right) people
Listen to the feedback of your employees who carry out the processes on a daily basis. They know exactly what information they need, and, in particular, what information is not needed.
Do not leave the revision of the QMS to one person, e.g., the QMR: Only the process owners themselves can adequately describe their processes and carry out risk management for them.
Tip 4: Streamlining the QM system
Eliminate unnecessary documents and content: The QM system should not contain content that is not required by regulatory authorities and does not contribute to risk minimization.
Implementing the entire Section 7 of ISO 13485 is also often unnecessary. The processes should be tailored to the current and future planned in-house IVD.
5. Summary and conclusion
a) Summary
There are various templates for medical laboratories that you should use when creating a QMS. The minimum requirement is legally prescribed and fulfilled by complying with Rili-BÄK Part A.
Almost all medical laboratories also use in-house IVD medical devices for their analyses. This means that the IVDR requirements must be met. These include implementing a QMS according to ISO 15189, which must be extended to include additional content from ISO 13485.
b) Conclusion
The IVDR’s requirement for an extended system based on ISO 15189 means that more laboratories are aligning themselves with this standard, and other QM standards will become less prevalent in the laboratory sector.
The required expansion of the system to include processes for development, manufacturing, modification, and monitoring can lead to excessive demands. Laboratories should, therefore, find the right balance between meeting the strict requirements of the IVDR and maintaining flexibility and efficiency in their day-to-day work.
The Johner Institute team helps medical laboratories implement or expand their QMS as the law requires. We ensure that the QM system remains lean and practicable and supports daily routine.
With our seminars, workshops, and e-learning offers, we have the right support for everyone to help laboratories become more efficient in their daily work.
Contact us to find out more.


