Manufactures use the “Research Use Only” (RUO) label to declare that their products should not be used in diagnostic procedures. This enables them to avoid the time-consuming and costly documentation required for conformity-assessed in vitro diagnostic medical devices (CE-IVDs). Nevertheless, some medical laboratories still use RUO products in diagnostic procedures, sometimes even with the knowledge of the manufacturers. This can have consequences – not just for manufacturers and operators but for patients as well.
In this article, you will learn
- what the “Research Use Only” (RUO) label means,
- what the requirements for RUO products are,
- how to avoid legal problems, and
- what alternatives there are to RUO products.
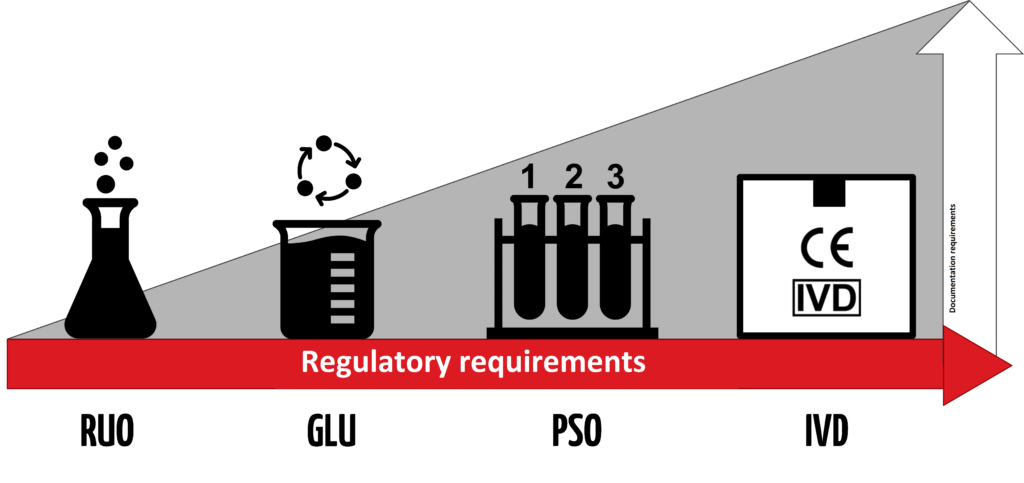
1. “Research Use Only” – what does it mean?
Products labeled “For Research Use Only” are hardly subject to any regulatory controls. Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) aims to distance itself from RUO products clearly:
The scope of application of this Regulation should be clearly delimited from other legislation concerning products, such as medical devices, general laboratory products and products for research use only.
IVDR Foreword (7)
a) Institutions affected
The following institutions, in particular, use RUO products:
- Medical laboratories
- Medical laboratories can utilize RUO products, but doing so designates them as the manufacturer, carrying all the associated consequences.
- If medical laboratories utilize RUO products for purposes beyond research, this can potentially render them liable for damages and subject to criminal liability in the worst-case scenario.
You can find more information on “Lab Developed Tests” in our article The EU regulates medical laboratories – Are Laboratory Developed Tests still allowed?
- Manufacturers
- Manufacturers can incorporate RUO products as components in their IVD, but they are subsequently responsible for ensuring the conformity of the end device with the IVDR. The RUO labeling of the component is not mandatory.
- If manufacturers designate their devices as “RUO,” the intended use of these devices must be interpreted accordingly and, if required, substantiated. For instance, reasonably foreseeable misuse should be taken into account. The RUO label should not be applied to the device as a mere “protective claim,” as this may result in legal consequences.
b) Definition
There is no standardized definition for “Research Use Only” (RUO) products. Generally, they can be understood as products designed for analysis intended solely for scientific research purposes, as the name implies. Their main distinction from medical devices lies in their inability to be used for medical purposes.
Nevertheless, the interpretation of “Research Use Only” varies between Europe and the USA.
Definition in Europe
In Europe, currently the MDCG 2024-11 guidance document provides a basis for a definition of RUO products and refers to the IVDR. The document serves to clarify the qualification of products as IVD medical devices or as accessories of IVD medical devices.
MDCG 2024-11 states:
“Products intended for research use only cannot be intended by their manufacturers for a medical purpose (as outlined in Article 2(2) IVDR).”
Source: MDCG 2024-11
Hence, an RUO product shall not have a medical purpose, not even a rudimentary one.
This also applies to tests developed in-house (Home Brew Tests, Laboratory Developed Tests) that are only used in a health institution for research purposes.
The IVDR addresses RUO products in Article 1 (3) a).
The IVDR excludes RUO products from its scope:
This Regulation does not apply to:
(a) products for general laboratory use or research-use only products, unless such products, in view of their characteristics, are specifically intended by their manufacturer to be used for in vitro diagnostic examination;
Source: IVDR, Article 1 (3) a)
Furthermore, Article 2 (45) specifies:
“A device intended to be used for research purposes, without any medical objective, shall not be deemed to be a device for performance study;”
IVDR, Article 2 (45)
Devices for performance studies are:
“‘device for performance study ’ means a device intended by the manufacturer to be used in a performance study”
IVDR, Article 2 (45)
The IVDR thus distinguishes RUO products from IVDs and products for performance studies. The EU regulation also highlights the lack of a medical intended purpose for RUO products.
Definition in the USA
In 2013, the FDA published a guidance document on RUOs entitled “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only.”
This guidance defines RUO products as follows:
“An RUO product is an IVD product that is in the laboratory research phase of development and is being shipped or delivered for an investigation that is not subject to part 812”[NB: Part 812 concerns the provision of devices for performance evaluation purposes as a preliminary step to IVDs]
FDA guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”, Chapter III A
Some examples of products that the FDA believes fall into this research phase of development are:
- Tests that are in development to identify test kit methodology, necessary components, and analytes to be measured.
- Instrumentation, software, or other electrical/mechanical components under development to determine correct settings, subcomponents, subassemblies, basic operational characteristics, and possible use methods.
- Reagents under development to determine production methods, purification levels, packaging needs, shelf life, storage conditions, etc.
However, the FDA further specifies:
“FDA also recognizes that there are certain products, such as instruments, systems, and reagents that are labeled for research use only and intended for use in the conduct of nonclinical laboratory research with goals other than the development of a commercial IVD product […].”
FDA guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”, Chapter III A
And subsequently gives examples of such research purposes in which the product itself is not the subject of research.
The FDA thus sees two “types” of RUO products: First, IVD devices whose development is ongoing and which are themselves the subject of the research purpose, and second, products for nonclinical research.
In both cases, the FDA requires a clearly visible RUO label to be affixed to the products. The RUO label is intended to prevent use for clinical diagnostics, patient management, and other investigations with a medical purpose.
c) What are the consequences of using the “Research Use Only” label?
Normally, IVDs are subject to regulatory requirements (for example, according to the IVDR or FDA) based on their risk class.
However, RUO products do not fall within the definition of “in vitro diagnostic medical devices” given by the IVDR or the relevant FDA regulations. This means that these regulations do not apply to RUO products.
‘In vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state;
(b) concerning congenital physical or mental impairments;
(c) concerning the predisposition to a medical condition or a disease;
(d) to determine the safety and compatibility with potential recipients;
(e) to predict treatment response or reactions;
(f) to define or monitoring therapeutic measures.
Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices.
Source: Article 2 IVDR
“In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.”
Source: 21 CFR 809.3
However, RUO products do not automatically fall entirely outside the regulatory scope in the EU. Depending on the product, they may still have to comply with requirements that are not specifically intended for IVDs (such as the REACH regulation for chemicals or the Machinery Directive).
Read more about the Machinery Directive: Which parts apply to medical devices.
Since RUO products are subject to considerably fewer controls than IVDs, it is necessary to severely restrict their use. Therefore, in particular they may not be used to
- make diagnoses and
- conduct performance studies.
2. Use and misuse of “Research Use Only” labels
a) What should RUO products be used for?
As the name “For Research Use Only” indicates, products with RUO labeling are intended for research purposes only. RUO products are particularly attractive for the research sector due to the simplified process and lower hurdles for placing them on the market.
The guidance document MEDDEV. 2.14/2 rev.1 (IVD guidance: Research Use Only products; A Guide For Manufacturers And Notified Bodies) provides a precise list of areas where RUO products may potentially be used. Although this guideline was developed under the now outdated Directive 98/79/EC on in vitro diagnostic devices (IVDD), it can still be considered state of the art in the absence of a current replacement (MDCG Guidance Document Status: Ongoing):
- basic research
- pharmaceutical research
- better identification and quantification of individual chemical substances or ligands in biological specimens
- in-house manufacturing of so called “Laboratory Developed Tests” for research purposes
And of areas where the use of RUOs is expressly not permitted:
- use of raw materials which are labeled “For Research Use Only” but which are incorporated into a finished product
- so called “research use products” being tested against a comparator IVD product that bears the CE mark
- products for market studies/feasibility studies
These products can be assigned a medical purpose.
b) What RUO products are often used for
However, the low hurdles are also the reason why RUO products are often used for purposes they are not intended for. This poses significant dangers for manufacturers, operators, and patients.
Sale of RUO products to medical laboratories
RUO products are sold by manufacturers to medical laboratories. Although doctors sometimes also conduct research, this is not really the main purpose of a medical laboratory.
Therefore, when discussing sales with doctors, it should always be assumed that there is a medical reason behind the use of the product. This means that anyone who knowingly sells RUO products to medical laboratories is potentially under suspicion of using the pretext “For Research Use Only” to ignore an intended medical purpose and thus avoid responsibility for a medical device.
Avoid reference to any specific diagnostic procedures in your advertising materials for products that clearly do not have a medical purpose. You should always stay on the technical or purely analytical level.
Use of RUO products in medical laboratories
The issue of selling RUOs to medical laboratories is not limited to manufacturers alone. The laboratories themselves may also not be acting in line with their status as operators and may, as a result, be liable under certain circumstances.
- Medical laboratories are free to develop in-house tests themselves. In such cases, RUO products are often used in diagnostic procedures. The laboratory bears full responsibility for these tests.
Even under the IVDD, MEDDEV 2.14/2 saw this topic critical. However, with the IVDR, the EU is explicitly placing more restrictions on the routine use of such Lab Developed Tests.
Read more in our article The EU regulates medical laboratories – Are Laboratory Developed Tests still allowed?
- Due to the low regulatory hurdles, purchasing RUO products is very affordable. As a result, medical laboratories prefer them over expensive CE-IVD devices if they can achieve the same level of performance. Nevertheless, the use of RUO products for purposes other than research, even in cases where they provide similar results, is not permitted.

The use of RUO/general laboratory use components in a diagnostic workflow may be covered by the intended use if an IVD manufacturer explicitly provides for the combination in the intended purpose/IFU and demonstrates safety, performance, and compatibility for that combination (e.g., extraction + PCR cycler/software + CE-marked IVD PCR kit)—either as a defined combination (closed system) or via critical specifications for permissible combinations (open system).
Team‑NB (association of Notified Bodies for medical devices) emphasises that if a manufacturer includes such products in combination claims, the manufacturer bears full responsibility for providing the supporting evidence; in addition, performance should be continuously monitored through Post-Market Surveillance (PMS). Where available, CE-marked instruments/components should be preferred.
3. Consequences of incorrect classification
Lack of controls can have a negative effect on quality. As a result, the relevant authorities (e.g., authorities during inspections) take a closer look at whether a product is actually intended “For Research Use Only.”
Manufacturers should also be aware that simply sticking an RUO label on a product does not on its own mean that the product no longer has to comply with requirements for IVDs that would otherwise apply.
The RUO status is determined solely by the actual intended use of a device. To this end, authorities (both European and FDA) also use marketing material or other information as evidence.
Manufacturers and operators who misuse the RUO label could face severe penalties, as such behavior can cause serious harm to patients or even the general public.
a) Consequences for manufacturers and operators
Improperly selling IVDs with an RUO label or using RUO products for purposes other than research is not a trivial offense. Manufacturers who intentionally conceal or attempt to conceal a diagnostic purpose behind the RUO label should anticipate legal consequences in Germany. The same applies for operators who misuse RUO products. There is the possibility of a fine or even prison sentences. In addition, there is potential liability for harm suffered by patients.
b) Consequences in the USA
There are also severe penalties in the USA. If an RUO label is deemed to have been incorrectly used for a product, the product would be considered misbranded under sections 502(a) and 502(o) of 21 US Code, 352(a), 352(o) [A1] and would be considered adulterated under section 501(f) of 21 US Code 351(f).
c) Consequences for patients
However, the consequences can be even worse for patients. After all, the regulatory requirements for IVDs aren’t just plucked out of thin air to annoy manufacturers and operators. The regulations are intended to protect patients against incorrect results and subsequent wrong decisions. False-negative results can lull patients into a false sense of security and an existing undetected disease may worsen. One example would be the metastasis of an undetected cancer due to a test not performing as intended.
Some incorrect diagnoses could even be so severe that they can cause the death of a lot of people: an undetected viral infection can cost many lives in the early stages of an epidemic or pandemic, as the coronavirus pandemic sadly demonstrated.
4. Alternatives to “Research Use Only” products
To avoid legal problems and risks to third parties, manufacturers and users should use general laboratory equipment as an alternative to RUO products.
There are laboratory products that obviously have no specific medical purpose, such as
- pure chemicals,
- culture media,
- reaction vessels,
- buffers,
- washing solutions,
- qPCR cycler,
- sequencers,
- centrifuges.
Read more on the topic here: General laboratory equipment: What manufacturers and laboratories need to know to avoid problems and unnecessary expense
5. Ways to protect yourself
Manufacturers, operators, and patients can take the following steps to avoid legal and other negative consequences when using RUO products:
a) Manufacturers
In the case of manufacturers, it is particularly important that they narrowly define the intended purpose of their product.
Analyte specific reagents should only be labeled as RUO products for specific non-medical purposes.
SARS-CoV-2 and its mutations: a test kit that uses specific primers and probes to distinguish the variants B.1.1.7 (alpha variant) and B.1.351 (beta variant) from the initial variant following a positive result may be an RUO product if it is only intended to be used to determine the prevalence of the variant in the population.
A specific intended purpose in this case would be: “Intended solely for epidemiological research for the purpose of surveying the prevalence of SARS-CoV-2 variants in the general population.”
If a medical laboratory subsequently, based on new findings, used this test to provide the best possible treatment for infection by a specific variant, this would be an off-label use. The laboratory would then be responsible for the test’s conformity.
Tip: Provided the manufacturer did not advertise the product with this clinical benefit, it would be adequately protected.
b) Operators
Operators should record exactly for what they use IVDs and RUO products.
Medical laboratories are operators of medical devices and IVDs and, therefore, are responsible for only using medical devices according to their intended purpose and in accordance with the generally accepted rules of the technology. This is stipulated in Section 4 of the German Medizinprodukte-Betreiberverordnung (MPBetreibV).
To be on the safe side, laboratories should keep a record of which medical devices and IVDs are in operation and routine use. This record should include a reference to the applicable test procedure and the intended purpose of the IVD.
This record can also be used to identify investigational procedures for which there are no adequate CE-IVDs available on the market. The lack of alternatives would justify the use of RUOs in validated processes as in-house IVD, provided that the laboratory verifies and demonstrates that the general safety and performance requirements and the additional requirements of Article 5(5) of the IVDR are met.
Read more about the requirements for LDTs in our article.
c) Patients
Patients lack the knowledge to recognize what is and isn’t an RUO on their own. They are often given little to no information about the test they are undergoing. So, patients should follow this basic rule: ask your doctor or pharmacist!
- Patients can ask for the complete test report from the laboratory so that they can get a second opinion in case of doubt. The report should also indicate which specific test was performed.
- Patients should inform themselves about how “well” or “poorly” a test works, as well as the benefit-risk ratio.
- In the future, patients and doctors will also be able to get information about medical devices from EUDAMED and use this information to decide whether or not the test was performed with certified and thus legally compliant IVDs.
6. Conclusion
In the opinion of the EU Commission and the FDA, products “For Research Use Only” have no place in diagnostics. To be used for diagnostic purposes, products have to go through the necessary controls. But these controls do not apply to RUO products.
Anyone who ignores this prohibition and uses or sells RUO products for purposes other than pure research is playing with fire. Manufacturers and operators run the risk of legal trouble and could even endanger patients’ health. Therefore, RUO products should only be used for research purposes. For other uses, manufacturers and operators should use the alternatives mentioned.
If you, as a manufacturer or medical laboratory, find that an RUO product is particularly well-suited for in vitro diagnostics, consider whether further development and conformity assessment to make it an IVD is worthwhile.
Thanks to Dr. Boris Handorn, lawyer and partner at PRODUKTKANZLEI, Augsburg, for his valuable input on this article.
Benefit from the support of our IVD experts:
- They will help you qualify your devices or examination procedures, for example, with in-house workshops on approval strategy and in-house IVDs.
- They provide you with expert opinions on the qualification of your device, which you can submit to your customers and/or notified bodies.
- They support you in all activities up to the “certification” of your device (e.g., performance evaluation) and beyond (e.g., post-market surveillance).
Or use our e-learning platform: Learn how to meet the regulatory requirements and get access to our IVD-specific templates and tutorials on how to get your device approved.
Change history
- 2026-02-18 Section 2(b): use of RUO in the diagnostic workflow supplemented; reference added to the Team‑NB position paper
- 2024-10-11 1. b) Reference to MDCG 2024-11 added, MEDDEV 2.14/2 rev. 1 removed as source; 2. a) 2nd section explanation of MEDDEV 2.14/2 rev. 1 added
- 2024-02-01 Complete revision; section “The thing with analyte-specific reagents” removed; shortening of chapter 4 (deletion of subchapters a) to c)); reference to article on general laboratory equipment
- 2021-11-16 First publication



I cannot access the specific URL you provided. However, if it’s about “For Research Use Only (RUO)” in regulatory affairs, a comment could emphasize the critical role of clear labeling and compliance in ensuring safety and integrity in research settings, promoting transparency and trust in scientific practices.
Hi RRMA Global,
Thank you for the comment! There seems to have been a mismatch in the links. These should now all be correct.
Kind regards
Tea Bodrusic
I did not find anything about use of RUO labeled materials in high school labs. Would a manufacturer distribute RUO labeled materials to high school labs?
Yes, a manufacturer may distribute RUO‑labeled materials to high school laboratories if they are used solely for non‑diagnostic educational or research purposes, with labeling and promotion that clearly state “For Research Use Only. Not for use in diagnostic procedures.”
Both FDA and EU IVDR frameworks permit this, provided there is no implication of clinical use and all applicable safety, handling, and labeling requirements are met.