An FDA registration is a legally required registration of “establishments” (such as medical device manufacturers) in an FDA database. The FDA registration should not be confused with the UDI registration in the GUDID database or even an FDA approval.
This article answers the most important questions and helps to avoid the most common mistakes in FDA registrations.
1. Who needs to register?
1.1 Definition “establishment”
The FDA requires the registration of “establishments” in 21 CFR part 807.
“a place of business under one management at one general physical location at which a device is manufactured, assembled, or otherwise processed”
In other words, it is about the actors involved in the manufacturing or processing of a medical device.
The FDA registration concerns the actors and their locations. It must not be confused with the listing, which concerns the devices.
Registration is, therefore, similar to the registration of economic operators under the MDR via the “Actor Module” of EUDAMED. However, significantly more actors are affected by FDA registration than under the MDR (see 1.3).
1.2 Activities in the manufacturing and processing of medical devices
It is helpful to have an overview of the activities involved in the manufacturing and processing of medical devices in order to understand and identify the roles involved.
- Typically, manufacturers determine the intended use of the future device and
- identify the stakeholder requirements.
- In most cases, manufacturers also specify the devices, e.g., their user interface, and
- they develop the device; for example, they design the system architecture.
- Typically, manufacturers also test the device and design output.
- For production, components and parts must be purchased or produced in-house.
- These are assembled and tested in the finished medical device.
At the end, specifications, produced components (e.g., circuit boards) or the complete medical device are available.
1.3 Division of tasks into roles
These activities can be carried out by the manufacturer itself or can be outsourced. This would be unusual for the intended use definition but is more common in the design and development of the device. Development service providers offer these services.
A manufacturer can also outsource production, both of components and of the medical device itself.
If the manufacturer has components produced by a third party according to its specifications, the FDA refers to this as a component manufacturer.
For the production of entire devices, the FDA distinguishes between:
- contract manufacturers
- contract sterilizers
- contract packagers
For example, if the manufacturer has the complete device manufactured externally according to its specification and then sells it under its own name, the manufacturer is a specification developer for the FDA in this case.
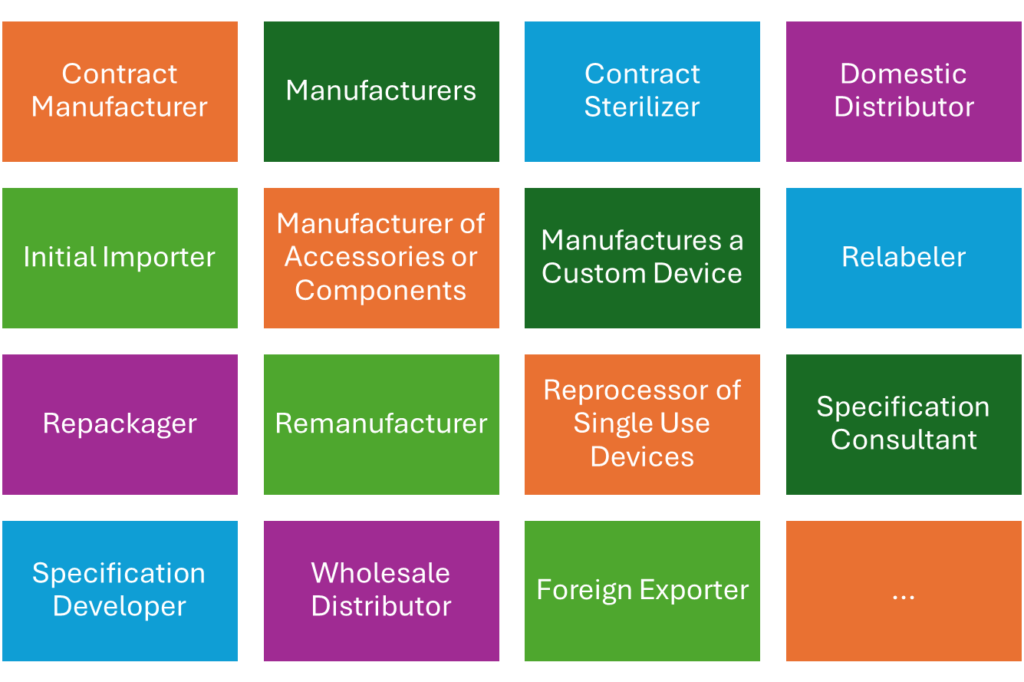
There are other roles, e.g., the “initial importer” or the “relabeler”.
During MDSAP audits, the establishment registration is reviewed, as is the correct assignment of roles. Deviations are regularly identified.
1.4 Obligation to register
Not all roles have to register their “establishments.” Tab. 1 provides an overview.
| Activity | Register domestic establishments | Register foreign establishments |
| Contract Manufacturer (including Contract Packagers) | YES 807.20(a)(2) | YES 807.40(a) |
| Manufacturers (including Kit Assemblers) | YES 807.20(a) | YES 807.40(a) |
| Contract Sterilizer | YES 807.20(a)(2) | YES 807.40(a) |
| Device being investigated under IDE | NO | NO 812.1(a) |
| Domestic Distributor that does not import devices | NO 807.20(c)(3) | — |
| Initial Importer | YES 807.40(a) | — |
| Maintains complaint files as required under 21 CFR 820.198 | YES | YES |
| Manufacturer of accessories or components that are packaged or labeled for commercial distribution for health-related purposes to an end user | YES 807.20(a)(6) | YES 807.20(a)(5) |
| Manufacturer of components, that are not otherwise classified as a finished device, that are distributed only to a finished device manufacturer | NO 807.65(a) | NO 807.65(a) |
| Manufactures a custom device | YES 807.20(a)(2) | YES 807.20(a)(2) |
| Refurbishers or Remarketers of used devices already in commercial distribution in the United States | NO | — |
| Relabeler or Repackager | YES 807.20(a)(3) | YES 807.20(a)(3) |
| Remanufacturer | YES | YES |
| Reprocessor of single use devices | YES 807.20 | YES 807.20(a) |
| Specification Consultant Only | NO | — |
| Specification Developer | YES 807.20(a)(1) | YES |
| U.S. Manufacturer of export only devices | YES 807.20(a)(2) | — |
| Wholesale Distributor that is not a manufacturer or importer | NO | — |
| Foreign Exporter of devices located in a foreign country | –– | YES 807.40 (a) |
An “establishment” can take on several roles, and there can be several “establishments” for one role.
2. When must the FDA registration take place?
The initial registration and listing of devices for domestic establishments (i.e., US establishments) must take place no later than 30 days after placing them on the market.
For foreign establishments, registration and listing must take place prior to import. Otherwise, the shipment may be detained by customs.
In addition, registration must be renewed, which requires annual re-registration. The annual registration must take place at the beginning of each FDA fiscal year, i.e., from October 1 to December 31.
Updates are possible anytime during the year and are necessary if changes occur.
3. What is the procedure for FDA registration?
The FDA provides guidance. The most important steps in the registration process are:
- First, an annual fee must be paid via the relevant FDA website. This is $7,653 for the current FDA fiscal year 2024 (October 1, 2023, to September 30, 2024).
- You will receive a Payment Identification Number (PIN) and, after successful confirmation of receipt of payment, a Payment Confirmation Number (PCN). This is required for registration.
- The actual registration takes place in the FDA Unified Registration and Listing Systems (FURLS), more precisely in the “Device Registration and Listing Module (DRLM).”
- To do this, the “owner/operator” of the respective establishment must first create an account. The “official correspondent” is responsible for the initial and annual registration. This role can also be assumed by an external person. A separate sub-account of the type “official correspondent” must be created in FURLS for this purpose.
- When registering, administrative data such as the establishment’s name, address, contact person, and other contact information must be entered. For foreign establishments, the US agent and the initial importer must also be entered.
The data on the devices must then be listed in the same FURLS module. This includes the trade name, risk class, product category or code and reference to the approval (e.g. 510k number).
A step-by-step guide at CDRH Learn is helpful: Click on the “Start Here/The Basics! (Updated Module 10/16/2023) / MDUFA Small Business Program, Registration and Listing”. An “accordion” will open, in which you will find presentations about registration in the “Registration and Listing” section.
A document guides you through all the registration and listing module input masks.
4. How long does an FDA registration take?
The actual registration takes place directly (after confirmed payment). This typically takes 1 to 2 hours.
Listing the devices can take a little longer, depending on the number of devices to be registered.
5. What typical mistakes should be avoided?
Mistake 1: Annual(!) registration completed too late
The first mistake is the establishment forgetting to complete the annual renewal of their registration within the deadlines. It can then happen that the FDA deactivates the registration. In this case, devices may no longer be sold.
Mistake 2: Incorrect assignment and incorrect data
Examples:
- Establishments are assigned an incorrect role during registration. For example, you register as a “contract manufacturer” even though you only manufacture one medical device component and are therefore only considered a “component manufacturer.”
By registering as a “contract manufacturer”, the establishment (depending on the device) falls under the Quality Management System Regulation (QMSR) and there is the possibility of an inspection by the FDA. - It can happen that a foreign manufacturer selects the wrong importer during the registration process.
- When the company address (or the official correspondent) changes, you may forget to update this information. To prevent this, it can be useful to establish a corresponding procedure in your QM system.
Most of the data is publicly accessible in the FDA database, and therefore also available to competitors.
Mistake 3: Missing devices
It regularly happens to the “establishments” that they do not list all devices (including accessories) that are sold in the USA. This can lead to ordered recalls and warning letters in the worst case.
Mistake 4: No additional registration in the GUDID
In contrast to EUDAMED, there are two different databases for listing devices and registering UDIs in the USA: the FURLS/DRLM and the GUDID. Sometimes, manufacturers or “labelers” forget to additionally register the UDIs of their devices in the GUDID.
6. Summary and conclusion
FDA registration should not be confused with UDI registration. Both are mandatory steps that the FDA explains in detail.
The number and variety of roles are unfamiliar for companies that want to bring devices to the US market for the first time.
It is important that companies do not forget the annual registration, enter the data correctly, and keep it up to date. Standard operating procedures – and the Johner Institute – can help with this.
The Johner Institute supports medical device manufacturers and other stakeholders with FDA approval and all interactions with the FDA.
It can assume the role of official correspondent. The experts at the Johner Institute then take care of the initial and annual registration and listing of your devices. This means you don’t have to worry about doing anything wrong or missing deadlines.
The Johner Institut also helps to check and correct individual entries. It also takes on the role of the US agent.
Contact us for free, non-binding advice on how to get your devices onto the US market quickly and safely.