The FDA MAUDE database provides information on the “Manufacturer and User Facility Device Experience.” It thus corresponds roughly to the database used by the BfArM to publish manufacturer reports on risks.
Content of the FDA MAUDE database
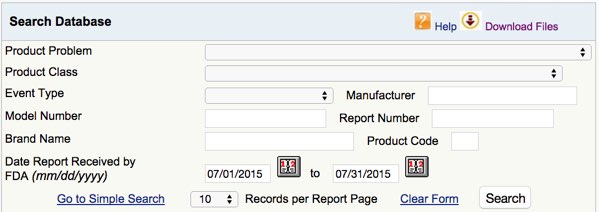
The FDA MAUDE database contains information on
- the problem
- the device class and device code
- the effect of the incident (death, injury, …) (“event”)
- the manufacturer
- the date of notification
Regulatory background
Obligation to evaluate
Manufacturers are obliged to conduct adequate risk management. It includes monitoring the device on the market as well as similar products and technologies. Only by doing so can manufacturers succeed,
- to identify hazards as completely as possible,
- to estimate probabilities of harm and problems, and
- to assess the severity of harm as correctly as possible.
The BfArM databases and the FDA MAUDE are among the sources you should always involve – regardless of the judicial area you market your devices in.
Obligation to report
In the USA and Europe, manufacturers and users must report problems and incidents. The FDA writes in this regard:
- Manufacturers and importers must submit reports when they become aware of information that reasonably suggests that one of their marketed devices may have caused or contributed to a death or serious injury or has malfunctioned and the malfunction of the device or a similar device that they market would be likely to cause or contribute to a death or serious injury if the malfunction were to recur. Manufacturers must send reports of such deaths, serious injuries and malfunctions to the FDA.
- Importers must send reports of deaths and serious injuries to the FDA and the manufacturer, and reports of malfunctions to the manufacturer.
- Device user facilities include hospitals, outpatient diagnostic or treatment facilities, nursing homes and ambulatory surgical facilities. Device user facilities must submit reports when they become aware of information that reasonably suggests that a device may have caused or contributed to a death or serious injury of a patient in their facility. Death reports must be sent to the FDA and the manufacturer, if known. Serious injury reports must be sent to the manufacturer or to the FDA, if the manufacturer is not known.
Change history
- 2023-12-06: Reference to Maude Analyzer of the Johner Institute removed


