The term “clinical validation” is also frequently used in the context of medical devices. For example, the German Federal Ministry of Education and Research (BMBF) has published a guideline on the clinical validation of innovative medical technology solutions (only available in German). The FDA also refers to clinical validation.
What is clinical validation? What distinguishes it from a clinical evaluation and a clinical investigation? Are clinical validations mandatory?
Here are the answers in a nutshell.
1. Definition and delimitation
a) Definition “clinical validation“
There are many definitions of the term, but unfortunately, no universally accepted one exists. Publications such as the one by Shah et al. are trying to overcome this.
Useful definitions
Most useful of all is this definition
Process based on clinical data to demonstrate that a medical device/IVD achieves its intended purpose and is safe and effective in doing so.
This definition considers the generally accepted definition for the term “validation” which is also used in ISO 14155-2021-05.
Confirmation through investigation and the provision of objective evidence that the requirements for a specific intended use can be met permanently.
Clinical validation is, therefore, a validation based on clinical data.
Less useful definitions
The following definitions are less common and less suitable:
| Definition | Assessment |
| Process of demonstrating a device’s ability to produce results that correlate with a specific clinical condition or physiological or pathological process or state in a specific target population and intended users | This definition is found in the context of IVD. It corresponds to the demonstration of clinical performance (and not, for example, analytical performance). |
| The process of testing a device’s accuracy to meet clinical standards (source) | This definition focuses on the device’s accuracy and does not include, for example, its usability, which should also be (clinically) validated. |
| Clinical validation is the process of validating each diagnosis or procedure documented within the health record, ensuring it is supported by clinical evidence in the medical record (source) | This definition is not appropriate in the context of medical devices. |
b) Differentiation from “clinical evaluation”
Clinical validation is part of the clinical evaluation: Clinical evaluation is also a process that includes the planning and realization of clinical validation.
During clinical evaluation, the data from the verification and validation of the device are evaluated. This may include clinical and non-clinical data.
Examples of clinical data are data from
- clinical investigations of the medical device,
- clinical investigations of comparable or predicate devices,
- scientific literature on comparable or predicate devices,
- post-market surveillance activities
Examples of non-clinical data include the results of biocompatibility tests.
Please note the article on clinical data.
c) Differentiation from “clinical investigation”
Clinical validation is based on clinical data. Clinical data are obtained in the context of the use of the medical device and consist of the following: data from clinical investigations with the device or comparable devices, published specialist literature on clinical experience with the device or a comparable device, or clinically relevant information from post-market surveillance.
The clinical investigation may be part of the clinical validation of a medical device to generate clinical data to demonstrate safety and performance. The term “validation” is often used in the context of clinical studies/investigations. For example, the HTA Guidance on Validity of Clinical Studies explicitly includes medical devices and IVD.
A clinical investigation should meet the following criteria to be considered “valid”:
- The clinical investigation should ensure that the results are clinically relevant and scientifically valid with regard to the endpoints of the clinical investigation and the benefit-risk profile of the investigational device.
- The design of the clinical investigation should be chosen so that conclusions can be drawn as to whether the investigational device is suitable for the patient population intended by the manufacturer within the scope of its intended purpose.
The document Guidance on Validity of Clinical Studies provides guidance in the form of definitions and explanations of different study designs, the strengths and weaknesses associated with the various designs, and the consideration of real-world data and real-world-evidence.
The guideline provides a practical framework for assessing the validity of clinical study results. It focuses on the definition, classification, and assessment of the safety of outcomes of clinical interventional studies, including medicinal products, medical devices, and IVD.
The guidance document covers various study designs and discusses their strengths and weaknesses, including Randomized Controlled Trials (RCT), single-arm studies, cohort studies, case-control studies, and non-traditional study designs such as master protocols (P, basket, and umbrella trials), and clinical registries.
d) Differentiation from non-clinical validations
Non-clinical validation is validation based on non-clinical data. Examples are the biocompatibility tests mentioned above and the validation of sterilization processes.
You can find more information about validation and the methods used under the keyword “validation.”
In most cases, non-clinical validation is carried out based on established (non-clinical) test methods described in standards. Examples of such standards can be found in the ISO 10993 and IEC 60601 families of standards.
The purpose of a bedpan washer is to clean and disinfect bedpans to interrupt the chain of nosocomial infections.
Full verification is provided by the associated product standard ISO 15883-3.
The device has no clinical outcome parameters to demonstrate its safety, performance, and benefits. Performance is provided by the A0 value (a measure of killing microorganisms in disinfection processes using moist heat). The acceptable limit values are listed in ISO 15883-3.
2. Legal requirements for clinical validations
a) Europe
Neither the MDR nor the IVDR use the term “clinical validation.” They demand a clinical evaluation (Article 61 MDR) or a performance evaluation (Article 56 IVDR).
Apart from the exceptions in Article 61(10), the demonstration that the device complies with the general safety and performance requirements must be based on clinical data assessed in the clinical evaluation. Collecting this clinical evidence corresponds to clinical validation.
b) USA
The FDA has numerous requirements considering “clinical evaluation.” However, it uses the term “clinical validation” almost exclusively for Software as a Medical Device (SaMD).
In the guidance document Software as a Medical Device (SAMD): Clinical Evaluation, the FDA makes it clear that it wants the term “clinical evaluation” to be understood in the same way as for IVD (see Fig. 1). Accordingly, clinical validation is the demonstration that the (correct and precise) output of the SaMD is suitable for actually achieving the intended purpose of the device in a clinical context.
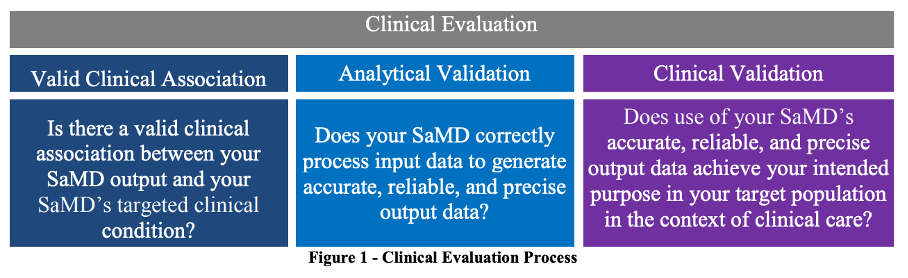
The FDA describes it as follows:
Clinical validation measures the ability of a SaMD to yield a clinically meaningful output associated to the target use of SaMD output in the target health care situation or condition identified in the SaMD definition statement.
Evidence of this is to be provided based on clinical data (see chapter 1.b of this article).
3. Procedure for clinical validations
In the clinical evaluation plan, manufacturers must plan the clinical strategy for the clinical evaluation and thus, if necessary, also the collection of further data as part of the clinical validation.
First, an “inventory” of all existing clinical data on the device is created. The existing clinical data is evaluated, and any data gaps that need to be closed as part of the clinical validation are identified. The clinical validation can, but does not have to, take place as part of a clinical investigation.
Clinical validation is carried out either before approval as part of a clinical investigation to generate clinical data for the conformity assessment procedure or in the post-market phase, for example, to confirm new clinical claims.
4. Conclusion and summary
The term “clinical validation” is not particularly helpful when it comes to medical devices and can lead to confusion. Medical device manufacturers should instead be familiar with the following terms:
- clinical evaluation
- clinical investigation
- (collection of) clinical data
By contrast, manufacturers of IVD and SaMD need to demonstrate clinical validity. The process of providing this evidence can be referred to as clinical validation.
The clinical experts at the Johner Institute help manufacturers of medical devices and IVD collect and evaluate clinical data to achieve quick and easy “approval” of their devices worldwide.
- They carry out clinical evaluations or provide support.
- They train the manufacturers’ employees, e.g., in a seminar.
- They create a clinical strategy for and with the manufacturers to avoid unnecessary expenses and surprises.
- They help with the implementation of clinical studies.
Please feel free to contact us, for example, using the contact form.

