Accessibility refers to the design of products and services that can also be used by people with physical limitations. The term “products and services” encompasses both physical and digital products and services. This also includes medical devices (physical devices, apps, other standalone software).
This article explains which accessibility requirements manufacturers of medical devices should be aware of to avoid the growing number of lawsuits.
Find out how a visually impaired person “experiences” a website.
1. Accessibility: What it is about
a) Definition “accessibility“
The German Equal Opportunities for Persons with Disabilities Act defines the term “accessibility” as follows:
“Barrier-free/accessible are buildings and other facilities, means of transport, technical objects of use, information processing systems, acoustic and visual information sources, and communication facilities, as well as other designed areas of life, if they can be accessed and used by people with disabilities in the usual way, without any particular difficulty and, in principle, without outside help. The use of aids necessary due to disability is permitted in this context.”
Source: Behindertengleichstellungsgesetz
Watch the video below to see how someone with disabilities experiences a website using a screen reader.
b) Scope of accessibility
Accessibility is, therefore, by no means limited to buildings: it is about more than just ramps and elevators for wheelchair users. Rather, it is about enabling as many people as possible with disabilities to participate in life – preferably without the help of third parties.
Barrier-free access should be provided for the following groups, for example:
- People with disabilities (especially with sensory and motor impairments)
- The growing number of older people
- The sick and injured
- People with “other” intellectual, linguistic, or cultural characteristics
c) Consequences of poor accessibility
Whenever there are regulatory requirements, there exist people and institutions that violate them, as well as people who act against these violations.
The number of corresponding lawsuits in the US is continuously increasing – by 37% in 2016.
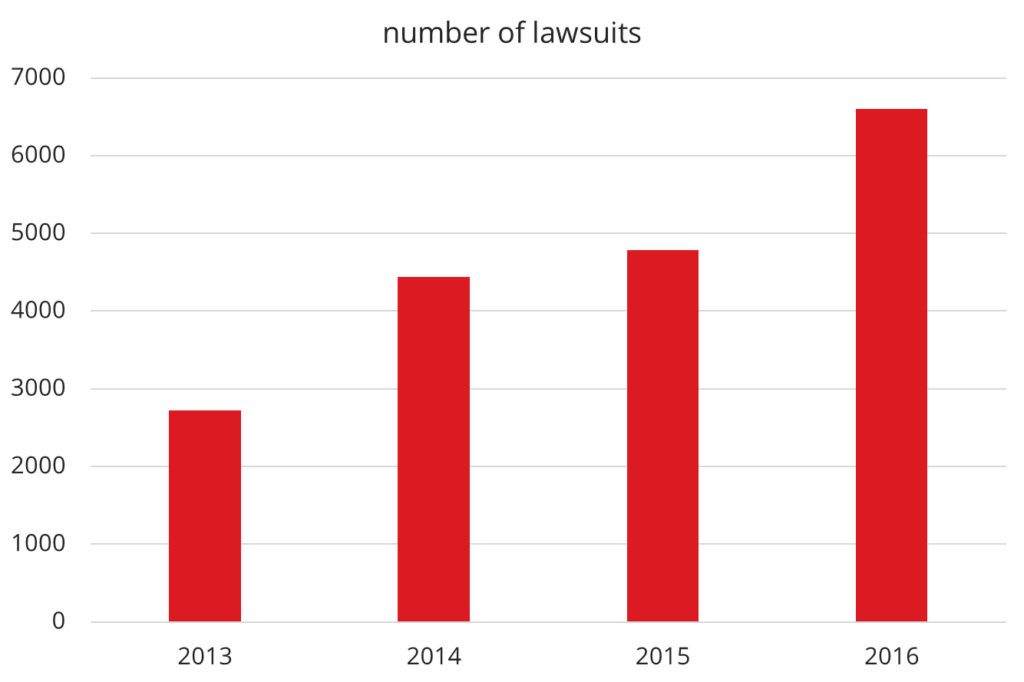
One of the main drivers of this growth is digital offerings.
As a medical device manufacturer, you should make your offers and products, such as websites and apps, especially those aimed at lay persons, barrier-free/accessible to avoid lawsuits.
2. Regulatory requirements for accessibility
a) Regulatory requirements in Europe
In 2019, the EU Directive 2019/882 “on the accessibility requirements for products and services” came into force. Manufacturers must comply with its requirements from June 28, 2025.
Affected products
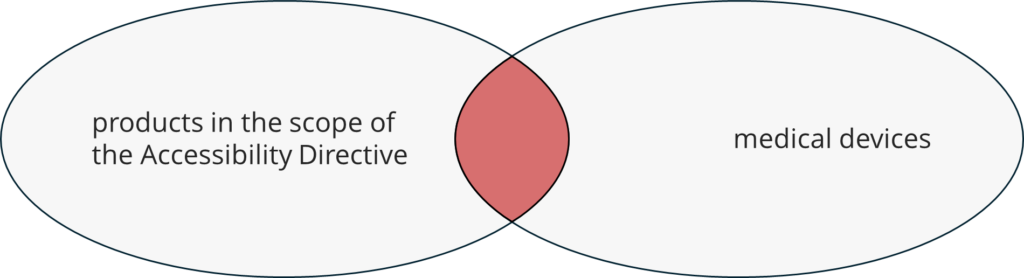
The EU has extended accessibility requirements to a number of products and services, whether provided by public entities or not. Most of these offerings do not include medical devices, such as ATMs, e-book readers, or banking services.
However, in individual cases, medical devices may fall within the directive’s scope.
| wording of the law | potential relevance for medical devices |
| interactive consumer terminal equipment used for electronic communications services | a system to support communication between physicians and patients |
| electronic communications services (according to Directive (EU) 2018/1972, these are, for example, internet access services or interpersonal communication services) | elements of the telematics infrastructure (if these count as medical devices) |
| responding to emergency calls made to the single European emergency call number 112 | systems for emergency call centers (if medical device) |
| e-commerce services | web-based health services that are offered or sold online |
The definition of “e-commerce services” is:
service provided at a distance, through websites and mobile device-based services, by electronic means and at the individual request of a consumer, with a view to concluding a consumer contract.
Many medical apps and web-based medical devices lead (implicitly) to a consumer contract and would thus fall within the directive’s scope.
Requirements
The requirements for accessibility are stated in the directive in Annex I. These include, for example:
- Information on the use of the product must be available via more than one sensory channel
- Adequate font and font size
- Alternative presentation of “non-text content”
- Comprehensible language
Implementation
The directive also provides suggestions in Annex II for meeting the requirements:
- Provision of electronic files which can be read by a computer using screen readers so that blind persons can use the information
- Consistent or clear and logical use of the same terms so that persons with intellectual disabilities can better understand them
- Provision of subtitles for instructional videos
- Option to enlarge text or a specific pictogram or to increase contrast so that visually impaired persons can perceive the information
- On a computer, not just an error signal but also a written text or image indicating the error so that deaf persons apprehend that an error is occurring
b) Regulatory requirements in Germany
Numerous laws and ordinances in Germany have the objective of increasing accessibility. These include:
- Barrierefreiheitsstärkungsgesetz (BFSG serves to implement the directive) – Accessibility Strengthening Act
- Gesetz zur Gleichstellung von Menschen mit Behinderungen (BGG) – Act on Equal Opportunities for Persons with Disabilities
- Barrierefreie-Informationstechnik-Verordnung (BITV) – Barrier-Free Information Technology Ordinance
- Luftverkehrsgesetz (LuftVG) – Air Traffic Act
- Personenbeförderungsgesetz (PBefG) – Passenger Transportation Act
For example, the BITV requires the following:
- Alternative CAPTCHAs must be provided
- For video files, text alternatives or an audio track with equivalent information must be provided
- Color must not be used as the only means of coding
- It must be possible to enlarge the text by 200% without loss of functionality or content
- The entire user product interface must be operable using only the keyboard
Other European countries have similar requirements. For example, the United Kingdom also requires accessibility in the Equality Act 2010.
c) Regulatory requirements in the USA
The most important law in the United States with regard to accessibility is “The Americans with Disabilities Act” (ADA). This law regulates the physical structures of state institutions and public spaces. However, the “lawyers” also extend the “public space” to the internet and thus to public websites such as those of airlines or online distributors.
In other words, the claim to accessibility is being extended to digital offerings. The revised Section 508 Guidelines also feel responsible for this. These regulations initially affect the digital offerings of state institutions. However, requirements for commercial/public offerings are also being derived from them.
Interestingly, the law references specific standards (e.g., those of the W3C).
3. Accessibility standards
a) Web Content Accessibility Guidelines (WCAG) 2.0 by W3C
The best-known and most referenced standard comes from the W3C consortium, which is also responsible for standardizing HTML, CSS, and XML. This standard is W3C’s Web Content Accessibility Guidelines, or WCAG 2.0.
Unlike the BITV (see above), they not only set requirements for usability without a mouse or for texts as an alternative to videos or images, for example. The WCAG uses over 60 aspects to specify how these requirements can be specifically implemented in web offerings.
The W3C even shows a before-and-after example demonstrating how to turn a lousy website into a good one.
b) ISO 9241
The ISO 9241 family of standards sets out requirements for the design of user interfaces. People with and without disabilities benefit equally from these. These include requirements for
- information presentation
- design of forms
- design of user interfaces for the WWW
- user guidance
- color representation
- dialog design
With the ISO 9241-171:2008, the ISO 9241 focuses on accessibility specifically for software. A new draft is currently being developed.
The section ISO 9241-110:2020 interaction principles (= dialog design) (on conformity to expectations) also requires:
5.3.2.2 The interactive system should respond according to the needs of the widest range of users and widest range of contexts of use.
EXAMPLE 1 An interactive system provides a screen reader to give users auditory information instead of visual information.
EXAMPLE 2 An elevator has two sets of controls at a different height to facilitate use by people of different stature as well as wheel chair users.
NOTE Supporting the needs of the widest range of users improves accessibility. Further information on accessibility is available from the following.
- ISO/IEC 29138-1 provides information about the user accessibility needs of the widest range of users.
- ISO 9241-171 provides guidance on software accessibility to meet the needs of the widest range of users.
- ISO 21801 provides guidance on cognitive accessibility to meet the needs of the widest range of users.
- ISO/IEC 29136 provides guidance on hardware accessibility to meet the needs of the widest range of users
c) Further guidelines
The BBC is strongly committed to the issue of accessibility and has published HTML guidelines and “Mobile Accessibility guidelines.”
d) Special requirements for a medical device (manufacturer)
IEC 62366-1 does not explicitly address the issue of accessibility. It “only” requires manufacturers to characterize users (including physical limitations) and design user interfaces accordingly. The same applies to the FDA and its Human Factors Guidances.
Medical device manufacturers should not only make their medical devices accessible (if relevant) but also their other offerings, such as websites.
4. Improving accessibility
a) Motivation
Medical device manufacturers should ensure that their devices are accessible in order to
- comply with the legal requirements for usability for people with disabilities (à la IEC 62366, FDA, MDD, MDR),
- meet the legal requirements for accessibility in general and avoid lawsuits,
- minimize risks for patients / safety when people with disabilities use the devices, and
- increase market success, for example, by ensuring that the growing number of older users can and want to use the device.
b) Knowing which tools people with disabilities use
There are numerous tools available to help people with disabilities use digital offers:
- JAWS screen reader
- NVDA screen reader (open source)
- VoiceOver screen reader (part of the iOS operating system)
- TalkBack screen reader (part of the Android operating system)
- Windows Magnifier screen magnifier (part of the Windows operating system)
- ZoomText screen magnifier
- Dragon Naturally Speaking voice recognition software (often used by people with limited mobility)
These tools require digital content to be designed as accessible as possible. For example, a screen reader requires that a website is structured with headings (h1 – h6). Tables for formatting, on the other hand, are counterproductive.
c) Checking accessibility
The following methods help evaluate the accessibility of products (including websites):
- Inspection with usability experts
- Visual inspection against the specifications, e.g., of the standards mentioned above (usability verification)
- Working without a mouse
- Working without a monitor and with a screen reader
- Tool-supported measuring of font sizes, colors, and contrast ratios
- Participatory observation of people with disabilities: This would be a usability validation (= summative evaluation) with representative users in a representative user context.
The inspection reports are usually available in the following form:
- Short report with a summary of the results and recommendations
- Detailed tabular presentation
- Number of the problem
- Observation, problem
- Severity
- Violated rule
- Recommendation
The Johner Institute tests the accessibility of (medical) devices in its usability labs in Germany and the USA. It provides quick and cost-effective assistance in identifying and solving problems and ensuring compliance with regulatory requirements.
d) Using tools
Our usability experts use the following tools, for example:
5. Conclusion
Medical device manufacturers must comply not only with specific laws for medical devices but also with regulations that apply to all products and offers. These include the requirements for accessibility.
In addition to your devices, you should also design other offerings, such as websites, in accordance with the law.
The requirements for accessibility are clearly designated and can be quickly tested and checked.
These tests also help quickly find and eliminate usability problems that are not specific to people with disabilities.
Change history:
- 2024-10-10: Section 3.b) on the ISO 9241 family rewritten, in particular to add the standards from the family of standards
- 2024-09-26: Article restructured, chapter numbers inserted, chapter 2.a) inserted (EU directive)
- 2017-03-21: First version created (thanks to Dana Douglas for valuable input)


