Regulations such as MDR and ISO 13485 require manufacturers to define quality objectives and a quality policy. These are prerequisites for the conformity of the QM system and thus for obtaining the associated certificate.
1. A hierarchy of quality policy, quality objectives and key performance indicators
Poor leadership leads to the failure of the entire organization. If management doesn’t know where it’s headed, then the rest of the organization won’t either. That’s why quality policy and objectives are not a QM system’s formalities; instead, they form the “North Star” by which the company aligns itself.
Unfortunately, many managers fail to formulate their quality policies and objectives precisely. These are not long texts. However, it takes some thought to determine the right ones for the company.
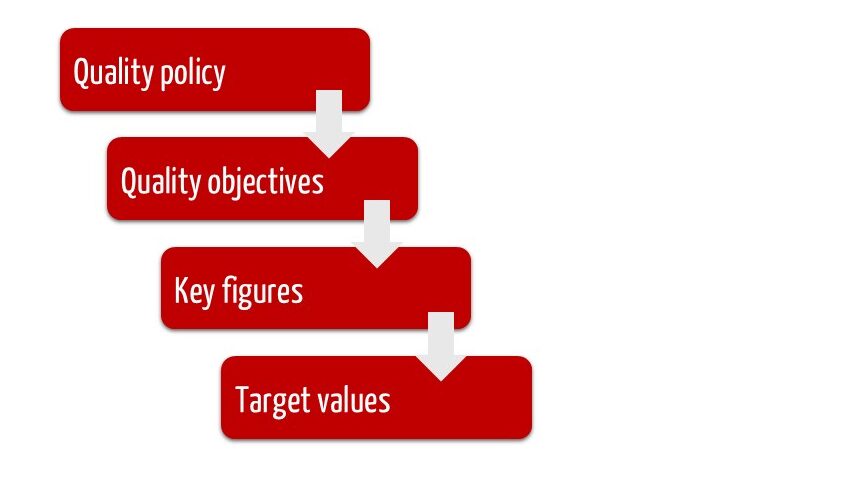
Companies should derive their quality objectives from the mostly constant quality policy and define key performance indicators to determine the degree to which these objectives have been achieved. Many companies change (usually tighten) the target values for these key performance indicators over time.
2. Quality policy
a) Definition
„Overall intentions and directions of an organization with regard to quality concerns, as formally expressed by the top management“
Source: ISO 9000
b) Meaning, translation, synonyms
Many people have difficulty with the term “policy.” In English, we speak of a quality policy, which could be translated as a (quality) strategy or as a (quality) guideline.
If you translate quality policy as corporate guidelines (or corporate policy), the meaning becomes clearer. The corporate philosophy, vision, and mission are also elements of a quality policy. Quality policy can also be understood as the big, overarching vision, an attractive picture of the future, and what the company aims to achieve in the long-term.
Often, companies formulate a quality policy that involves becoming the “leading provider…” in a particular field. However, this has little to do with quality in the sense of compliance with customer requirements. You will find better examples below.
c) Examples
Two examples may help to understand the term quality policy better:
- Manufacturer of medical devices for diabetics: This manufacturer’s quality policy is: We provide a comprehensive range of products and services that allow patients to be as little affected as possible by their diabetes. This quality policy addresses quality in its most original sense, namely the degree to which the characteristics of a product or service meet real requirements.
- Johner Institute: The Johner Institute formulates its quality policy in the form of a mission and the form of its values:
- Mission: The mission of the Johner Institute is to support manufacturers of active medical devices in developing and approving safe products quickly and professionally while avoiding or reducing quality management bureaucracy. You can read the complete mission here.
- Values: The Johner Institute’s values represent the guidelines and ethical corset for implementing the mission. These values include the constant pursuit of excellence and higher competence, taking care of the customer, and indispensable honesty and reliability.
The quality policy should be particular to the company. Below are examples of typical formulation mistakes.
3. Quality objectives
a) Definition
“Objectives with regard to quality”
ISO 9000
b) Meaning, synonym
Quality objectives are specific, measurable (using key performance indicators), and achievable (SMART) intermediate steps to ensure compliance with the quality policy. These objectives should be formulated more precisely than the quality policy. Many companies find it easier to delete the word “quality” from the term and only speak of objectives. These objectives can affect all areas of the company:
- The company as a whole: turnover, profit, new customers
- Processes: error rates, complaints, throughput times, process costs
- Products: number of new products, error rates, complaints
c) Hierarchy of quality objectives
Many companies find it easier not to derive specific (SMART) quality objectives directly from the quality policy but to formulate “high-level” quality objectives and then derive them from them.
d) Examples
This example is intended to illustrate the path from quality policy to “high-level” and specific quality objectives:
- Quality policy: We provide a comprehensive range of products and services that allow patients to be as little affected as possible by their diabetes.
- Quality objective (“high-level”): We develop a medical device that avoids painful blood sugar measurement and insulin injections.
- Quality objective (concrete): By the end of the year, we will have studied all relevant publications, patents, and products and evaluated all known alternatives based on a cost-benefit analysis.
Further examples can be found below.
4. Key performance indicators and target values
One or more key performance indicators should be defined for each quality objective to determine progress and the degree of achievement. Good key performance indicators are
- specific, i.e. they measure how well the quality objective is being achieved,
- easy to collect, i.e., without causing unnecessary effort, and
- easy to understand.
Example: Key performance indicators for the quality objective “We achieve our objectives with highly trained employees” would be the percentage of employees who have actually been trained in a given period, the number of certificates they have acquired, and the number of publications and conference papers that have been published by company employees.
Finally, a target value must be set for each key performance indicator, and it must be measured.
5. Typical mistakes when formulating quality policy and quality objectives
- The quality policy is abstract, dispassionate, and arbitrary. It neither mobilizes forces nor creates a shared sense of purpose. I frequently read documents that would make any narcotic completely superfluous. This belongs under the Narcotics Act! 😉
- The quality objectives are replaceable. Cut out everything that does not apply equally to every other equivalent company. What company does not want “high customer satisfaction”? If nothing remains after cutting out these banalities, throw the whole manual in the trash. QM systems must be built from the top down. And if the top is worthless, it will not be any better at the levels below.
- The QM manual confuses quality policy with quality objectives.
- Management commitment: The boss is not behind the QM system and even delegates the quality policy formulation to third parties, such as the QM representative. Such a QM system tends to resemble Potemkin villages.
- No continuous improvement of the QM system: While quality policy is usually a slow-moving target, you should continuously review the metrics (key performance indicators) and their target values: Are they still suitable for tracking the achievement of objectives? A few key performance indicators recognized by the team will help you improve quality more effectively than a static, ever-expanding management cockpit.
- Avoid costly mistakes when setting up your QMS with the right know-how.
- Discover our innovative e-learning platform that provides you with the necessary knowledge. With many examples and best practices from consulting, you will benefit from expert tips and implement your system error-free. Start now and build a quality management system that offers your company tangible added value!
6. Further examples
a) Examples of high-level quality objectives
- We develop products that enable cancer patients to be treated according to the latest scientific findings.
- In general, we solve critical errors in our software for our customers as quickly as possible.
- We increase our productivity by working with the latest technologies.
- From now on, we will also prepare our employees for their tasks by providing regular and comprehensive further training.
- We guarantee the best possible IT security for our customers’ data.
- We gain the trust of our customers through honesty and transparency.
These quality objectives must be translated into specific goals in the next step.
b) Examples of specific quality objectives
The high-level quality objectives should be broken down into particular, measurable, and scheduled objectives:
- We will solve all software errors that are either security-related or prevent work within two days and deliver updates within three days.
- By the end of the year, we will have five Product Managers trained as usability and requirements engineers.
- We will install a backup solution for all central servers and validate the associated process by the end of the quarter.
- We will revise our “Software Development Standard Operating Procedures” by the next release to enable agile implementation.
- By the end of the month after next, we will have processed entirely the CAPA list.
c) Examples of key performance indicators
Development
- Number of new patent applications
- Number of new projects
- Percentage of projects completed on time and on budget
- Duration to fix errors
- Number of complaints, bugs
Support
- Percentage of problems that can be solved directly in support
- Duration until issues are solved
- Number of contacts with the customer until the problem is solved
Sales
Sales is also often controlled by key performance indicators. Examples include the number of
- contact attempts,
- customer contacts,
- offers,
- contracts, and
- new customers.
Click this link to download the free starter kit, which provides an overview of the regulatory landscape and outlines the six steps to “approving” your medical device.
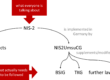
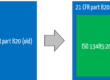
7. Regulatory Requirements
Companies are obliged to define a quality policy by
- ISO 13485:2016 Chapter 5.3,
- ISO 9001:2015 Chapter 5.2,
- FDA 21 CFR part 820.20 (“quality policy”).
The MDR and IVDR do not explicitly address quality policy
The obligation to define quality objectives is formulated by
- ISO 13485:2016 in Chapter 5.4.1,
- ISO 9001:2015 in Chapter 6.2,
- FDA 21 CFR part 820.20 (“quality objectives”),
- MDR and IVDR each in Annex IX, paragraph 2.2.
8. Quality objectives and QM manuals: support
Do you need support in
- formulating your quality policy?
- Deriving quality objectives?
- Defining key performance indicators and metrics?
- Writing a lean and precise QM manual?
- Establishing, testing, and certifying your ISO 13485-compliant QM system?
Then get in touch with us! We specialize in quickly setting up an ISO 13485 compliant QM system. Read on to find out how.
We look forward to hearing from you!
- E-mail: info[at]johner-institut.de
- Contact form: www.johner-institut.en/contact/
Change history
- 2025-05-07: Boxes with definitions reformatted; editorial changes to chapter headings 2 and 3
- 2020-10-05: Regulatory requirements added. Editorial changes, including chapter headings