Trade goods in the medical device sector can become a regulatory trap for distributors. The line between distributor and manufacturer under the MDR and IVDR is thinner than many people think, with potentially costly legal consequences.
1. Trade goods according to the MDR/IVDR
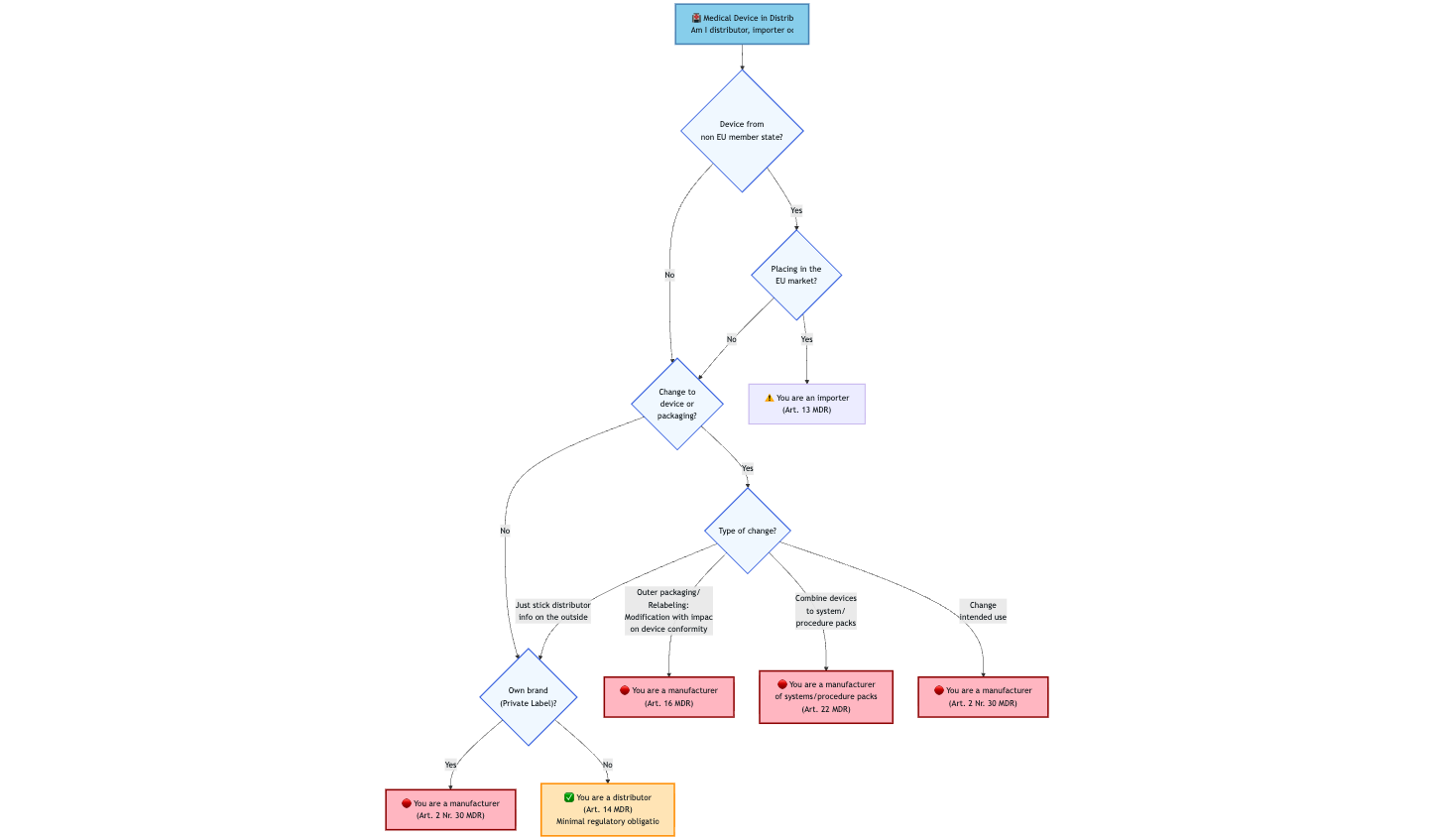
The MDR and IVDR do not contain an explicit definition of the term “trade goods.”
Trade goods refer to medical devices that
- already have CE marking,
- are purchased from the distributor or manufacturer and are resold in their original, unaltered condition.
That means that distributors act as pure transit points in the supply chain. The device thus retains its original manufacturer and the manufacturer’s responsibilities.
2. Delimitations
2.1 Delimitation from own brand
A (medical) device becomes a trade good if
- the distributor purchases it and resells it unchanged,
- no modifications are made to the device or packaging,
- the original label is retained, and
- the distributor adds at most:
- his own contact details as a distributor
- additional distribution information (outside the original packaging).
Distributors may resell trade goods under their own trade name or brand if this has been agreed in writing with the manufacturer and the manufacturer continues to be indicated as such on the product label. The responsibility for compliance with regulatory requirements, therefore, remains with the manufacturer.
However, the device is NO LONGER a trade good in the following cases:
- Private label: Own trade name or own trademark on a third-party product, original manufacturer not visible
- Change of intended purpose
- Modifications of any kind to the device or packaging that affect the conformity of the device
In these cases, the distributor becomes the manufacturer with all manufacturer obligations!
The MDR defines when modifications to the device are not considered to be an activity that affects the conformity of the device. Read more about this in the article on requirements for distributors (which also affect manufacturers).
Special case: Systems and procedure packs
Distributors who combine multiple products into a set become manufacturers of systems and procedure packs and fall under Article 22 of the MDR.
2.2 Delimitation of activities
The activities involved in trading medical devices determine the applicable legal obligations that the distributor must fulfill.
| Activity | Distributor sells the device under the manufacturer’s name or brand or under his trade name or brand, if agreed with the manufacturer | Distributor sells the device under his own name, trade name, or brand Distributor changes the intended purpose Distributor modifies the device or packaging in a way that affects the device’s conformity |
| Visibility | Original manufacturer remains on device Distributor contact details on label and/or only in shipping documentation | Name of the distributor on the device Original manufacturer not visible |
| Responsibility | Manufacturer retains full responsibility | Distributor takes on manufacturer responsibility and product liability |
| Regulatory obligations | Minimal (see below) | Full manufacturer obligations in accordance with Article 10, including technical documentation QMS in accordance with ISO 13485 Own CE marking Registration in EUDAMED |
3. Legal obligations
3.1 Requirements for distributors of trade goods
The MDR and IVDR set out the requirements in Article 14 (Obligations of distributors). These obligations include:
- Conformity assessment upon purchase:
- CE marking present
- EU declaration of conformity available
- UDI marking (if required)
- Instructions for use in the required languages
- Storage and transport conditions in accordance with the manufacturer’s specifications
- Random checks to ensure proper condition
- Storage of supplier/customer data (at least 10 years)
- Reporting obligations to manufacturers and authorities in case of incidents
- No separate registration in EUDAMED as a distributor required
The distributor must also contribute to achieving an appropriate level of traceability in the product supply chain. To this end, the distributor must be able to provide the competent authority with the following information:
- All economic operators from whom he has purchased a device and to whom he has supplied it directly
- All health institutions or healthcare professionals to whom he has supplied a device directly
3.2 Requirements for importers of trade goods
When dealing with trade goods, two roles must be distinguished:
Distributor (Art. 2 No. 34 MDR):
- Device already placed on the market in the EU
- No changes to the device/packaging
- Pure resale
- Example: Medical supply store purchases from German wholesaler
Importer (Art. 2 No. 33 MDR):
- Placing on the market in the EU
- Device comes from a third country
- Importer must be named on the device
- Example: Direct import from the USA/China
This article provides an overview of the obligations and tasks of importers.
4. Particularities of software as a medical device
Standalone software as a trade good has become rare. That is because manufacturers are increasingly marketing and operating the software directly.
Nevertheless, pure distributor status is still possible:
- Pure distribution of licenses or rights of use (e.g., through activation codes)
- Distribution of unmodified software on data carriers
The line between distributor and manufacturer can easily be crossed in the following cases:
- Distribution via download → placing on the market
- Hosting → provision
- Updates/patches → product modification
5. Conclusion and summary
The legal requirements for the distribution of trade goods are manageable. However, distributors must not only comply with these requirements but also ensure that they do not overstep the boundaries to become manufacturers or importers.
The Johner Institute answers questions from all economic operators (distributors, manufacturers, importers) and supports them in meeting regulatory requirements. Get in touch.

