Chemical characterization according to ISO 10993-18 is a central component of the biocompatibility evaluation according to ISO 10993-1 and, thus, a prerequisite for the approval of medical devices.
It is used to identify unknown substances in medical devices to carry out a toxicological risk assessment.
This article provides an overview of
- chemical characterization and its analytical methods,
- the legal requirements for medical device manufacturers,
- typical errors and best practices to achieve fast approvals and safe devices.
- FDA-compliant analyses
- Assistance with problematic test results
- Toxicological risk assessment
- Regulatory support for Q sub-meetings or questions from notified bodies
- Pragmatic and solution-oriented biocompatibility evaluation
- Strategic planning – streamlined and compliant
1. Chemical characterization according to ISO 10993-18
a. Definition
chemical characterization: process of obtaining chemical information, accomplished either by information gathering or by information generation, for example, by literature review or chemical testing
chemical information: qualitative and quantitative, if applicable, knowledge related to the configuration, composition and production of the medical device and/or its materials of construction, thereby establishing the identities and amounts of constituents present in the materials and device
b. Chemical characterization as process
Chemical characterization according to ISO 10993-18 is a process for ensuring chemical information for the biological evaluation of medical devices. Basically, three types of processes can be distinguished here:
- Proof of chemical equivalence: Comparison of a new medical device with an already approved device to determine equivalence
- Comparison with material standards: Review of whether the chemical composition of a device complies with the relevant standards and regulatory requirements
- Basis for the toxicological risk assessment according to ISO 10993-17: Identification and quantification of components of a medical device for the evaluation of biocompatibility according to ISO 10993-1
Please note the further information on the toxicological risk assessment according to ISO 10993-17.
2. Role of data sheets in chemical characterization
a. Which data sheets exist
Safety Data Sheets (SDS) – occupational safety and hazard assessment
Safety Data Sheets (SDS) provide important information, e.g., on the composition, safe handling, storage, and disposal of chemicals and mixtures. Raw material suppliers, manufacturers, or distributors are legally obliged to provide an SDS for:
- Hazardous chemicals: if the substance or a mixture or individual ingredients are classified as hazardous (e.g., irritant, toxic, dangerous for the environment)
- PBT or vPvB substances: substances that are classified as persistent, bioaccumulative, and toxic (PBT) or very persistent and very bioaccumulative toxic (vPvB) and thus have the potential to accumulate in an organism
- Substances of very high concern: substances on the candidate list for substances of very high concern in accordance with Article 59.1 of the REACH Regulation, e.g., CMR
The declaration of classified ingredients is based on regulatory requirements such as REACH (EC 1907/2006) and the CLP Regulation (EC 1272/2008) in the EU, as well as the Globally Harmonized System (GHS) worldwide. Accordingly, classified substances that make up at least 0.1% of the total weight must be declared.
Technical Data Sheets (TDS) – material properties and application data
Technical Data Sheets (TDS) contain detailed material and application specifications, including:
- Physical, mechanical, and thermal properties (e.g., temperature resistance, density, hardness, elasticity)
- Chemical resistance (e.g., resistance to chemicals and solvents)
- Composition, purity, and quality of the material or product
- Standards and certifications, application examples (medical devices)
A TDS is not required by law. However, many manufacturers provide one to minimize product liability risks and to convey information.
Certificates – regulatory compliance
Certificates are issued as proof of compliance with regulatory requirements.
- Biocompatible according to ISO 10993 series or USP Class VI
- Food grade
- Further
b. Why data sheets are not enough
Although SDS and TDS, as well as certificates, provide valuable chemical information, they are insufficient for a complete chemical characterization according to ISO 10993-18. The reasons for this are:
- Incomplete information on the chemical formulation: Manufacturers of raw materials, especially polymers, often do not provide detailed identities and quantities.
- No information on releasable substances: The chemical composition alone says nothing about which substances the medical device releases in its final state under clinical conditions.
- Dependence of the materials used: How the materials are processed, the additives used in production, and the aging or reaction of the materials with others affect which substances can be released.
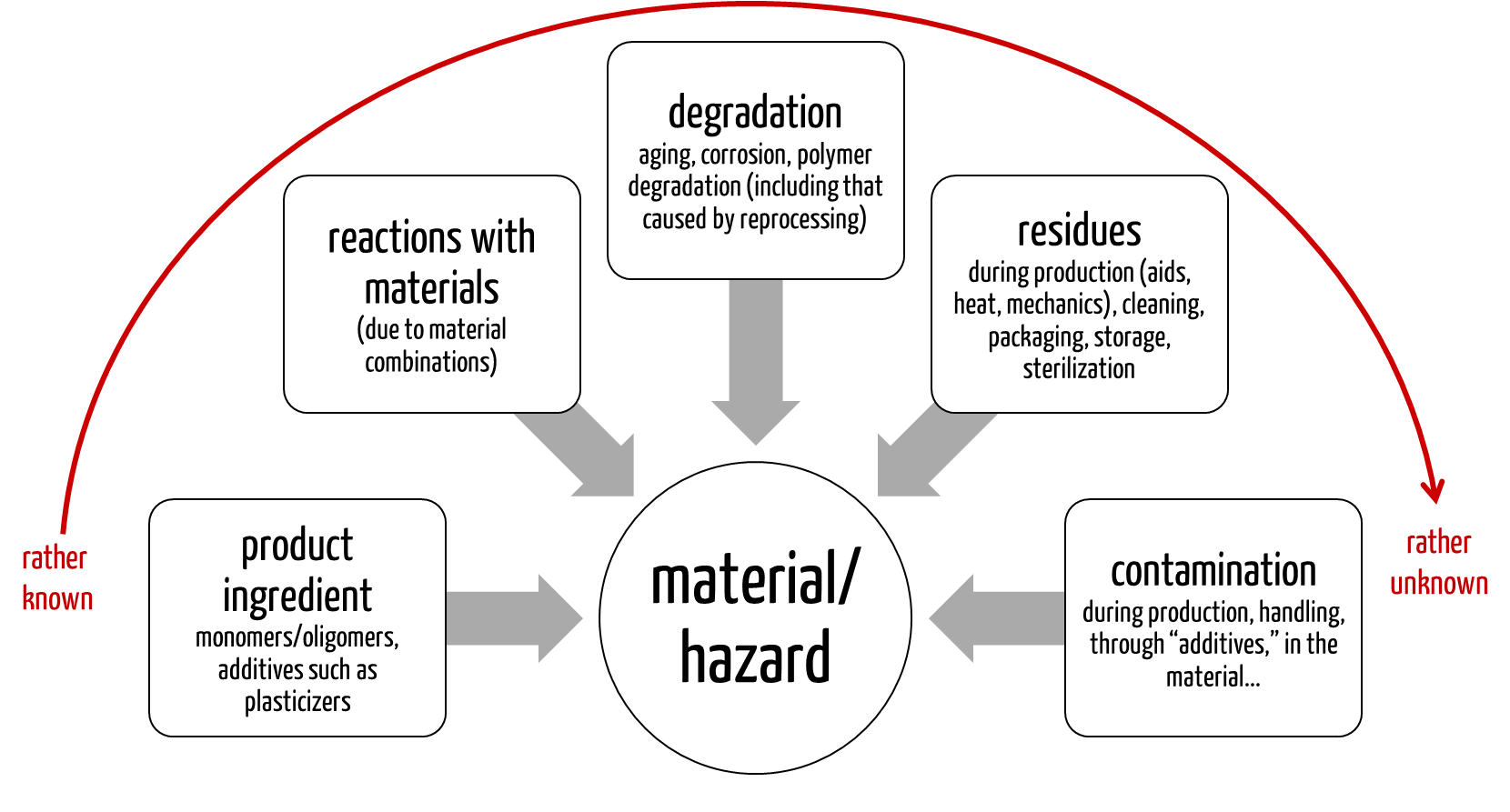
Since other factors besides the materials used can affect the possible release of toxicologically relevant substances and thus lead to a hazard, a chemical characterization of the final medical device using analytical methods is, in most cases, indispensable.
3. Chemical analysis: Implementation in accordance with ISO 10993-18
Risks-based approach
ISO 10993-18 defines the requirements for the chemical characterization and, specifically, the chemical analysis of medical devices as part of the biological evaluation. The objective is to identify and quantify releasable chemical substances or materials to estimate potential toxicological risks.
ISO 10993-1 requires a risk-based approach that considers the type and duration of patient contact. Chemical characterization plays a central role in efficiently adapting the test strategy and avoiding unnecessary animal testing.
Step 1: Extraction according to ISO 10993-18
The first step in chemical characterization is the extraction of the medical device. In this process, chemical substances are dissolved out of the material under defined conditions to evaluate which substances could be released during clinical use.
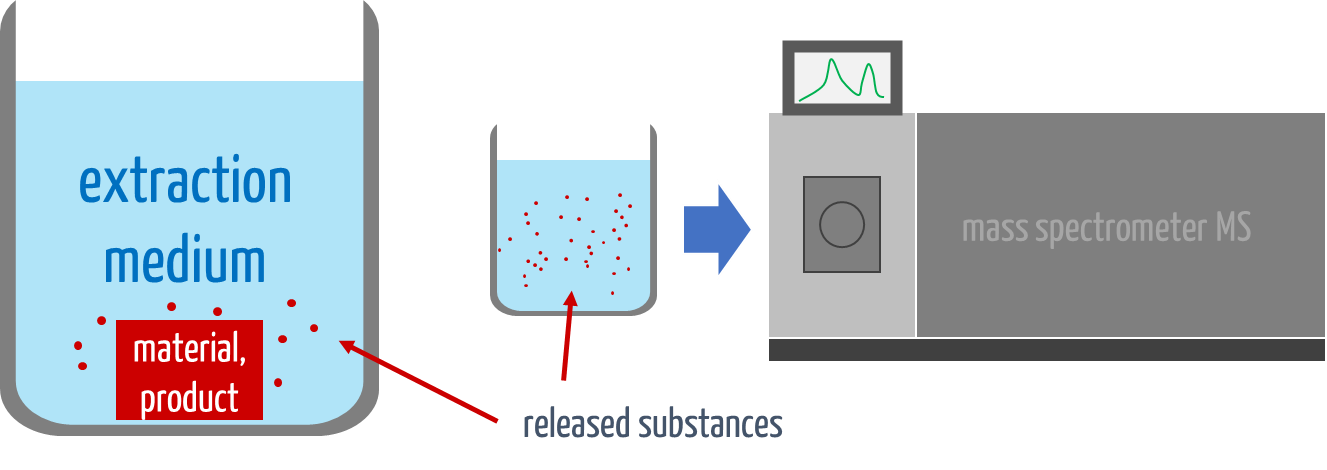
The type of extraction depends on the length of the contact time (see Tab. 1).
| contact category | recommended extraction conditions | plausible alternatives |
| limited contact | simulated use condition | exaggerated conditions |
| prolonged contact | exhaustive extraction | exaggerated conditions |
| long-term contact | exhaustive extraction | exaggerated conditions |
In addition to the contact duration, other factors must be considered when determining the extraction conditions:
- Cumulative application with new medical device
- Reusable medical devices
- Materials and release behavior, e.g., non-absorbable metal
- Solvent resistance of materials
Important extraction parameters that must be specified and justified are:
- Temperature
- Duration
- Type of extraction media (e.g., polar, semi-polar, non-polar)
- Product surface
Step 2: Determination of released substances using analytical methods
After extraction, chemical analysis methods are used to identify and quantify the released substances. The choice of method depends on whether the substances are volatile, semi-volatile, or non-volatile and whether they are inorganic or organic.
- Gas chromatography coupled with mass spectrometry (GC-MS): for the analysis of volatile and semi-volatile organic compounds
- High-performance liquid chromatography (HPLC) or liquid chromatography coupled with mass spectrometry (LC-MS): for the identification of non-volatile organic substances
- Inductively coupled plasma mass spectrometry (ICP-MS): for the detection of inorganic elements
These analytical procedures are crucial for detecting potentially critical substances that could enter the body during the use of the medical device.
The data obtained are directly incorporated into the toxicological risk assessment according to ISO 10993-17. A precise chemical characterization also helps to reduce the testing effort for biocompatibility studies and can contribute to avoiding animal testing.
ISO 10993-18 proposes test methods (see Tab. 2).
| type of substances | example procedure/method |
| volatile organic compounds | Headspace-GC-MS, GC-FID |
| semi-volatile organic compounds | GC-MS, GC-FID |
| non-volatile organic compounds | HPLC, LC-MS |
| inorganic elements | ICP-MS, ICP-AES |
The method must be selected based on the type and duration of exposure, the type of material, and its release behavior in order to ensure a reliable chemical characterization.
Step 3: Calculation of the Analytical Evaluation Threshold (AET)
The Analytical Evaluation Threshold (AET) defines the lowest concentration of a substance that must be reliably quantified to perform a toxicological assessment.
The objective is to identify potentially harmful substances and ensure no relevant risks are overlooked.
Significance of the AET
Manufacturers can use the AET to decide whether a toxicological risk assessment is necessary:
They must toxicologically assess substances with a detected concentration above the AET. In contrast, substances with concentrations below the AET are not considered safety relevant.
In addition, the AET determines the required sensitivity of the analytical methods.
Calculation of the AET
The AET is calculated from several parameters:
- A: number of products extracted
- B: volume of the extract
- C: number of products in clinical use
- DBT: dose-based threshold, e.g. TTC (can be found in the literature)
- UF: uncertainty factor

Make sure that the sensitivity of the analyses in the context of the planned method meets the requirements of ISO 10993-18. Take into account the uncertainty of the laboratory’s analysis.
Accurate calculation of the AET is crucial for identifying clinically relevant risks and ensuring compliance with regulatory requirements.
4. Regulatory requirements
a. Europe/MDR
The MDR requires the biological safety of the devices in Annex I. Although the MDR does not explicitly require chemical analyses, ISO 10993-1 and EN ISO 10993-18 (harmonized in Europe) are, considered and expected to be the state of the art.
b. FDA guideline – chemical analysis
The FDA guideline “Chemical Analysis for Biocompatibility Assessment of Medical Devices” was published in a draft version in 2024 and is already setting new standards for the planning, performance, and reporting of chemical analyses.
| contact time | extraction method | solvents | NVR analysis required? |
| < 24 h (limited) | exaggerated or worst-case condition | polar + non-polar | no |
| 1–30 d (prolonged) | exhaustive or exaggerated extraction | polar + non-polar | yes |
| > 30 d (long-term) | exhaustive or exaggerated extraction | polar, semi-polar + non-polar | yes |
The FDA considers ISO 10993-18 a “recognized standard”. In addition, it expects the following for chemical analyses:
- Testing of triplicates to take product variability into account
- Detailed documentation of sample preparation and analytical methods
- Standardized extraction solvents and procedures for dealing with material incompatibility
- Analysis of non-volatile residues (NVR) to demonstrate exhaustion of the extraction
- Comprehensive laboratory reports (calibration data, confidence level for identification, standards, etc.)
These requirements intend to ensure that the chemical analyses of extractables are reproducible, validated, and carried out in compliance with regulatory requirements.
5. Tips for avoiding errors
Errors in chemical characterization according to ISO 10993-18 can lead to delays in approval, unnecessary costs, and audit deviations. The following best practices help to avoid typical pitfalls and ensure an efficient biocompatibility evaluation:
Tip 1: Start planning early
- Material selection with a focus on biocompatibility: All chemical information on the materials used should be recorded during the material qualification process (material compliance) in the development phase.
- Involve experts: Early involving toxicology experts can help avoid unpleasant surprises later.
- Consider initial screening analyses: This allows potential risks to be identified early.
Tip 2: Define methods and parameters systematically
- Collect detailed chemical information: Identify the material composition and possible additives, considering potential degradation products and contaminants.
- Adopt a risk-based approach: The choice of analytical methods and parameters must be based on the type and duration of exposure and the material properties.
- Chemical analyses provide data for toxicological risk assessment, i.e., a successful risk evaluation depends on properly planned and executed chemical analyses.
Tip 3: Use validated analytical procedures
- Select a suitable laboratory: e.g., accreditation according to ISO 17025
- Prefer a laboratory with relevant experience in ISO 10993-18 and FDA guidelines
- Use the recommended validated methods according to ISO 10993-18
- Ensure the sensitivity of the analyses by AET determination
Tip 4: Perform a toxicological risk assessment according to ISO 10993-17
- The substances that can be released must be assessed toxicologically by an expert per ISO 10993-17; a chemical analysis alone is insufficient.
- A prerequisite for the risk assessment is a detailed identification and quantification of the substances.
- A conservative approach is necessary when determining the actual exposure, the tolerable intake, and the resulting margin of safety.
6. Conclusion and support
A well-thought-out chemical characterization according to ISO 10993-18 saves time and money and reduces the risk of regulatory hurdles. Manufacturers should develop a strategy at an early stage to:
- avoid missing or insufficient chemical data.
- efficiently plan test strategies and reduce unnecessary animal testing.
- choose test conditions and parameters wisely to ensure a successful toxicological risk assessment.
- implement FDA requirements for the US market now.
We will guide you through the entire process – from planning to approval:
- Strategy development in the Biological Evaluation Plan (BEP): risk-based test planning
- Organization and support for laboratory testing: ensuring valid analyses
- Plausibility check/measures for unexpected test results: troubleshooting for manufacturers
- Toxicological Risk Assessment (TRA): assessment according to ISO 10993-17
- Creation of the Biological Evaluation Report (BER): regulatory compliant documentation
Contact us for an efficient and standard-compliant chemical characterization according to ISO 10993-18!


