The Breakthrough Devices Program is an approval process for medical devices, and the FDA aims to enable seriously ill patients to gain faster access to novel medical devices. The authority published a “Guidance Document” in December 2018.
This article explains
- how the Breakthrough Devices Program works and
- which requirements manufacturers must comply with in order to participate.
1. Objectives of the FDA’s Breakthrough Devices Programm
The FDA wants to pave the way for manufacturers to get their medical devices to market faster. However, this offer only applies to novel products for patients with either a life-threatening illness or an illness that irreversibly weakens or impairs them.
2. Breakthrough Devices Program: The basics
The Breakthrough Program is not a new type of approval. It is, therefore, not an alternative to a premarket notification, according to 510(k), to a premarket approval or a de novo request. Instead, the FDA is using the new program to create the possibility of accelerating these existing approval procedures.
Read more about the Special 510(k) and Abbreviated 510(k) approval procedures here.
The program is open to medical devices and combination products. It replaces the following programs:
- Expedited Access Pathway
- Priority Review
- Innovation Pathway
3. How the FDA is expediting approval for breakthrough devices
3.1 The approach
The FDA promises several improvements to the manufacturers included in this program:
- Interactive communication
Manufacturers can interact directly with the FDA to clarify the clinical trial process or to discuss approval documents. - Higher priority
The FDA will process approvals (such as 510(k) and PMAs) with higher priority. The same applies to Q-submissions (e.g., pre-market requests). Applications for an Investigational Device Exemption (IDE) will also be processed with higher priority. The IDE is the approval to use the products for clinical studies. - Flexibility in clinical studies
Provided there are no safety concerns, the FDA allows some data to be collected only after the start of the post-market phase. The authority is prepared to accept temporarily higher risks in view of the high benefits (life-saving). The FDA allows the study design to be adapted during the clinical study and the approval process. - Dedicated, available, and well-trained review teams
The FDA provides sufficient and competent personnel. This competence also extends to the application of the Breakthrough Devices program. Even senior FDA management is on hand. - Quality management
The FDA may even disregard the quality management system by requesting fewer documents (“FDA may accept less quality system and manufacturing information”) or not insisting on a “preapproval inspection”.
This makes collaboration faster and more interactive – more comparable to an agile approach.
3.2 The Breakthrough Device Program procedure
The program runs in two phases. During the first phase, the manufacturer must submit an application in which they explain how they comply with the above-mentioned conditions. The FDA will decide on the application after 60 calendar days at the latest, and may request additional information after 30 days.
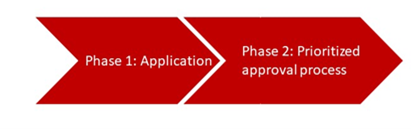
In the second phase, the FDA and the manufacturer work collaboratively in sprints. As part of this process, the FDA not only grants the manufacturer higher availability but also gives the “project” a higher priority than in other approval procedures.
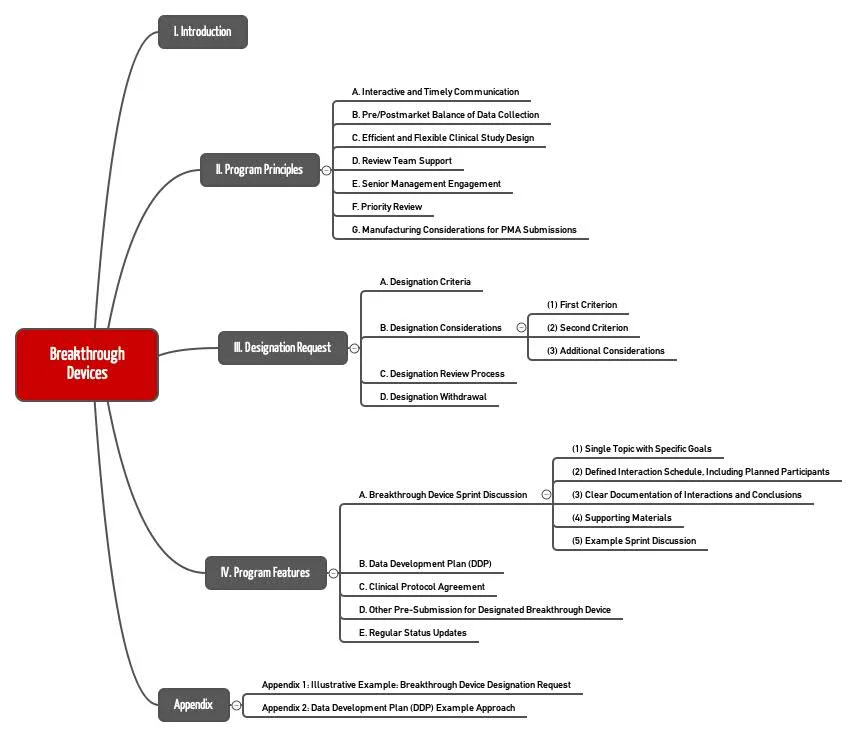
The FDA has issued a detailed guidance document that you can download here.
4. Prerequisite for participation
For a manufacturer to be able to have their product approved with the help of the Breakthrough Devices Program, they must comply with several requirements.
- The medical device offers better treatment or diagnosis of life-threatening or irreversibly damaging diseases or health conditions. Examples include stroke, heart attack, cancer, severe trauma, and ALS.
- At least one of the following criteria must be met:
- The device represents a technological breakthrough (Breakthrough Technology). An example would be a new genetic test that can be used to better weigh treatment options.
- There are no approved product alternatives. For example, there is currently no device that can diagnose Parkinson’s disease directly, according to the FDA.
- The product offers a significant improvement over approved alternatives.
- The product is in the best interest of patients. This would be the case if the alternatives had significant side effects or the new product significantly improved diagnostics and applicability..
If you are unsure whether your product can qualify for the Breakthrough Device Program, please get in touch. Our FDA experts in Germany and the US can quickly assess the situation.
5. Conclusion
Once again, the FDA has shown that it can evaluate the benefits and risks. They have recognized that denying or delaying the approval of medical devices can be just as harmful to patients as medical devices that are not sufficiently safe or effective.
A program like this would also be desirable in Europe. Unfortunately, Europe is taking the opposite approach under the MDR.
The Johner Institute supports medical device manufacturers worldwide in the approval process.
Our team assists you in selecting the appropriate approval procedure and supports you in communicating with authorities such as the FDA.
Register here if you want to market your medical device in the USA as quickly as possible and without unnecessary costs and effort.
Change history:
- 2024-02-25: Editorial changes; chapters renumbered and split
- 2019-01-15: First version of the article published


