The regulations relevant to usability engineering require formative evaluations.
Read more about summative evaluation in another article.
1. Formative evaluation: Definition and differentiation
a) Definitions
„User interface evaluation conducted with the intent to explore user interface design strengths, weaknesses, and unanticipated use errors.“
IEC 62366-1:2015 („Medical devices – Part 1:
Application of usability engineering to medical devices“)
In its guidance document, the FDA has published its own definition of the term:
„User interface evaluation conducted with the intent to explore user interface design strengths, weaknesses, and unanticipated use errors.“
Content of Human Factors Information in Medical Device Submissions
Thus, it replaces the old definition in the guideline Applying Human Factors and Usability Engineering to Medical Devices:
„Process of assessing, at one or more stages during the device development process, a user interface or user interactions with the user interface to identify the interface’s strengths and weaknesses and to identify potential use errors that would or could result in harm to the patient or user.“
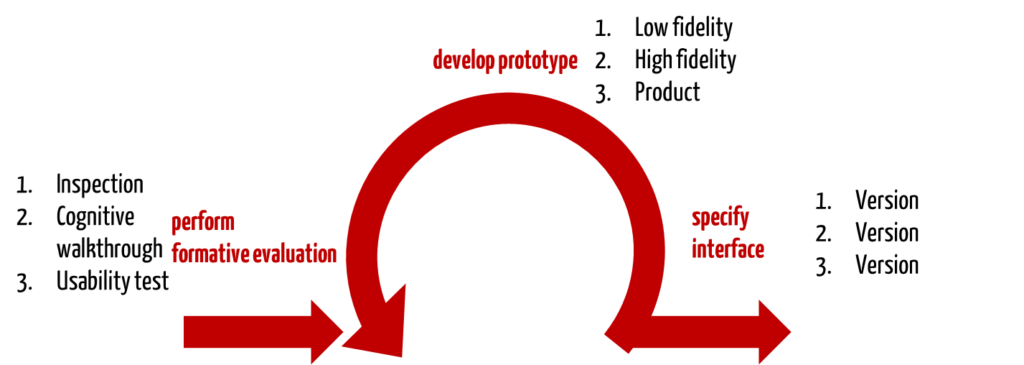
The formative evaluation must, therefore, be correctly understood as a development-accompanying evaluation of the user-product interface. It is carried out to discover the strengths and weaknesses of the user-product interface as well as unforeseen use errors.
Both guidelines are presented in this article.
b) Differentiation: Formative and summative evaluation versus verification and validation
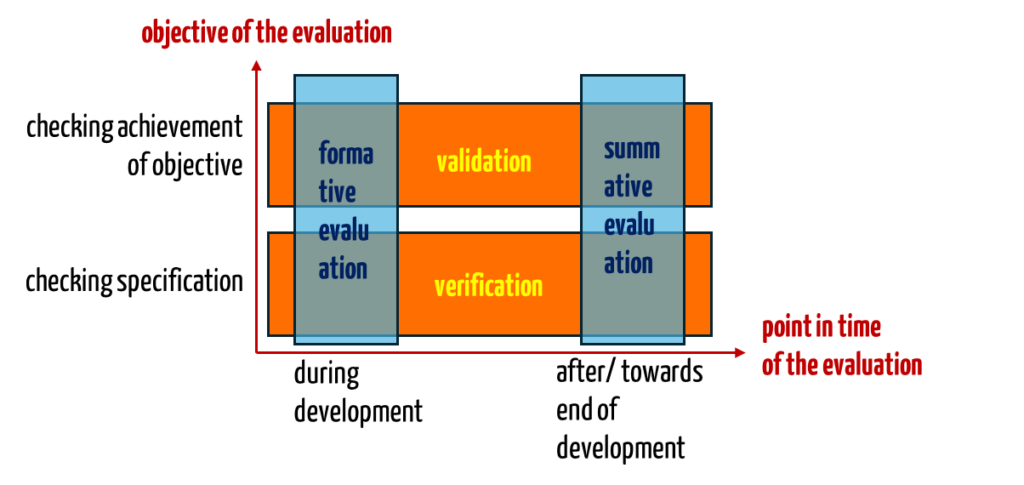
IEC 62366-1:2016 requires a formative (during development) and summative (final) evaluation instead of verification and validation of usability.
The two pairs of terms have little to do with each other – apart from the fact that they are all related to the usability inspection. Instead, the two pairs of terms refer to different aspects: Usability validation and usability verification differ in the objective of the inspection. On the other hand, the terms formative evaluation and summative evaluation describe the points in time of the inspection.
2. Formative evaluation: Regulatory requirements
a) IEC 62366-1:2015
IEC 62366-1:2015 requires the planning and implementation of formative (during development) and summative (final) evaluations.
Although the standard does not specify methods, it does require manufacturers to plan,
- which methods they will use for the evaluation (with a justification of the choice),
- at which points in time they will carry out these assessments,
- which parts of the user-device interface will be evaluated,
- which criteria they test, and
- with which users and in which user environment these evaluations are to take place.
Of course, these planning activities, the execution of the evaluation, and the results and their evaluations must be documented.
b) FDA
The FDA formulates its requirements in the guidance document “Applying Human Factors and Usability Engineering to Medical Devices.” Chapter 6.4.3 provides detailed information on formative evaluation.
Objectives
According to the FDA, manufacturers can use the formative evaluation to
- identify unknown use-related hazards, use errors, and critical tasks,
- implement changes to the user device interface in a timely manner,
- review the effectiveness of risk minimization measures on the User Interface (UI),
- determine the need for training measures and
- derive content for the validation plan.
Methods
The US authority does not require a specific method, but writes:
„Formative evaluation complements and refines the analytical approaches described in Section 6.3, revealing use issues that can only be identified through observing user interaction with the device.“
Thus, the FDA associates formative evaluation with methods in which users are to be involved and not with pure verification activities. The FDA does not exclude company employees as users but writes, “their performance and opinions could be misleading or incomplete.”
In the same document, however, it becomes clear that the FDA does not recognize participant observation as the only permitted method. The authority also mentions “cognitive walk-through, observation, discussion, interview.”
Planning
According to the FDA, the formative evaluation plan should specify:
- Objectives and priorities of the evaluation
- Elements of the user-device interface to be evaluated
- Use scenarios and tasks
- Participants/users
- Methods for data collection and data analysis
- How the results will be further used
These requirements are almost completely in line with those of IEC 62366-1:2015.
Documentation
The documents that should be created during the formative evaluation are defined in the FDA guidance “Content of Human Factors Information in Medical Device Submissions.”
Step by step to a legally compliant usability file
Most FDA recalls of software are due to usability problems. With our e-learning platform, you will understand the requirements of IEC 62366-1 and the FDA and learn how to create a legally compliant usability file step-by-step.
3. Methods for formative evaluation
Many methods are suitable for development accompanying (formative) evaluation. These include, for example
- cognitive walkthrough,
- verification, e.g., against style guides, heuristics, and design patterns,
- usability test (participant observation) with prototypes of different quality (low, high fidelity),
- interviews,
- questionnaires.
For formative evaluation, it is advisable to
- use several of these methods and
- to do this early, iteratively, and repeatedly in the development process.
Almost cynically, the FDA notes that formative evaluations should be used to identify usability problems at an early stage because otherwise the summative evaluation (the usability test) could fail; this would then itself become a formative evaluation because subsequent improvements to the user-device interface would be necessary.
4. Summary and conclusion
Formative evaluation is both mandatory and useful for medical devices. It helps to identify and eliminate usability problems more quickly and with less effort. As a result, manufacturers save money and bring their devices to market faster. There is a high probability that risks due to a lack of usability will have been sufficiently minimized.
Change history
- 2023-02-13: References to the new FDA guidance document regarding the content of approval documents added


after i read the IEC 62366-1 and FDA guidance ,there is no mandatory description for Formative evaluation
Dear Liam,
Thank you very much for your comment.
In chapter 5.7 of the IEC 62366-1 it says: “The MANUFACTURER shall establish and maintain a USER INTERFACE EVALUATION plan for the USER INTERFACE.”
And further: “the plan shall document the objective and identify the method of any planned FORMATIVE EVALUATIONS and SUMMATIVE EVALUATIONS;”
The FDA suggests in chapter 6 of their HFE guidance document a series of (formative) evaluation methods. The FDA also recommends in Annex A to give a “Summary of preliminary analyses and evaluations“ in the HFE report.
Although neither the IEC 62366-1 nor the FDA explicitly state that it is mandatory to conduct formative evaluations, it can be assumed that this is nevertheless the expectation of the regulatory authorities, as formative evaluations are an important process step in the development of safe medical devices.
I hope I could clarify. Please contact us if you have any further questions.
Best regards,
Nils