The Johner Institute supports medical device manufacturers, their suppliers, notified bodies, and authorities in all projects in a regulatory context. This includes transformation projects.
Transformation projects versus non-transformation projects
Projects either serve to run the business or change the business. The latter projects are transformation projects. Projects for the digital transformation of a company are particularly important transformation projects. The Johner Institute supports medical device manufacturers, their suppliers, notified bodies, and authorities in all projects in a regulatory context. This includes transformation projects.
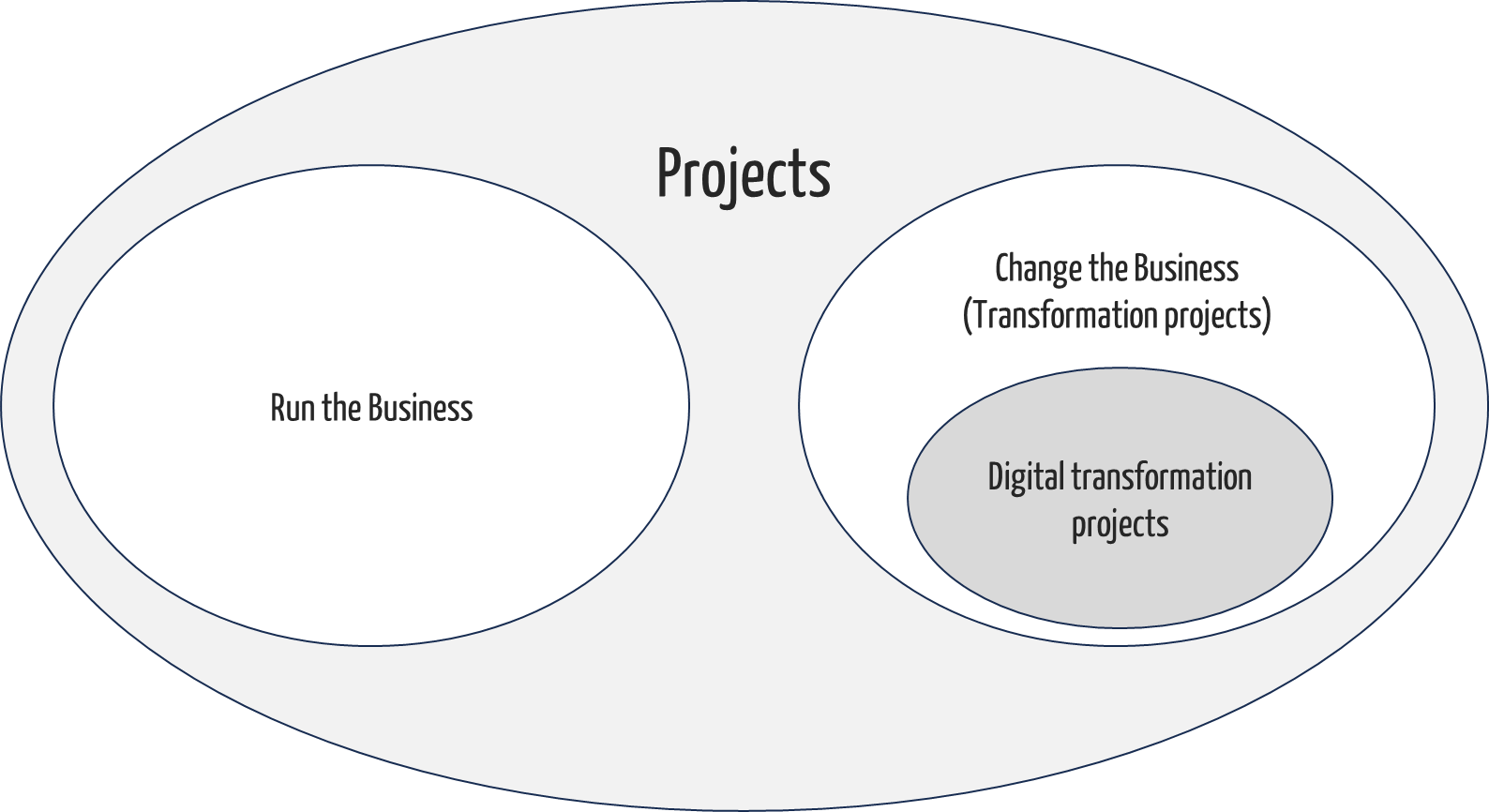
Some of the projects serve to “change the business”. This also includes digital transformation projects.
Digital transformation projects
Digital transformation is an ongoing process, as technologies and customer needs constantly evolve. Digital transformation projects have the following objectives:
- To radically reduce time-to-market and thus increase competitiveness
- To minimize regulatory risks
- To eliminate skills shortages, particularly in the regulatory area
- To free development from regulatory tasks
- To reduce costs for approval and regulatory affairs departments
While the process is ongoing, the projects are time-limited. They aim to achieve concrete outputs that contribute to the above objectives. Examples of these outputs are:
- Document-based approval has been converted to document-free approval.
- The review of technical documentation is automated.
- There is no more unstructured data in individual documents.
- Regulatory processes such as Post-Market Surveillance are automated.
- One or more data silos have been eliminated.
Further information
This article on digital transformation provides an overview of what organizations need to pay attention to.
Read here how to make digital transformation a success in your company.
Other transformation projects
The other transformation projects must also serve the company’s objectives, in particular to:
- increase customer benefits, e.g., through higher quality devices or better support
- improve conformity
- accelerate the efficiency of processes
- reduce overhead costs
- increase competitiveness
- improve employee satisfaction
Typical outputs of these projects are
- Processes are streamlined, e.g., unnecessary or redundant activities are eliminated.
- As a result, for example, the duration of development projects is shortened.
- Unimportant but time-consuming processes have been outsourced.
- Departments are organized along the value chain and not by function.
- The quality management system has been updated and successfully audited.
- Technical documentation is prepared in an “audit-proof” manner.
You will find the most essential starting points for transforming regulatory processes and organizations in the articles linked below.
Support from the Johner Institute
Do you have questions about the transformation of your company? You can get answers in our free micro-consulting.
Would you like to learn more about how your company can benefit from a transformation and what concrete steps you can take to make it a success? Contact us; we will be happy to support your transformation.
Companies need to master the digital transformation quickly and successfully. This also applies to medical device manufacturers because, after all, their future depends on it. Many companies make severe mistakes during this transformation (see section 4). This article provides managers with a quick overview of the possibilities of digital transformation and gives seven tips to make it…
Details
The digitalization of the production is an essential part of the digital transformation of manufacturing companies. However, not all companies benefit to the same extent from digitalization. This article describes,
Details
Medical device manufacturers have high expectations of Regulatory Information Management Systems (RIMS). The costs and efforts involved are immense and usually much higher than expected. The benefits, on the other hand, are not clear. This article will give you some hints,
Details
Many companies consider Regulatory Intelligence so important that they create their own roles and departments for it. This article clarifies what Regulatory Intelligence is, how companies can benefit from it, and where tools can provide support.
Details
The digital transformation of notified bodies will transform the medical device ecosystem over the next few years. This article describes
Regulators still publish laws and regulations as texts. Just as it has been for thousands of years. Regulation as Code represents a radical paradigm shift away from this practice. Is it possible to transform laws into algorithms? Why should anyone want to do this? How should you get ready for this as a regulator, manufacturer, authority, or…
Details
