“Follow the PICO scheme.” This is the recommendation of many auditors and reviewers, as well as some regulatory documents (such as MDCG guidelines and MEDDEV 2.7/1 rev.4), regarding precise medical literature searches.
Read in this article,
- what the PICO scheme is and which variants you should know,
- which tasks (including tasks other than literature searches) the PICO scheme can help you with, and
- how to successfully create and use a PICO scheme.
1. PICO scheme: The basics
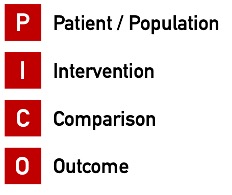
1.1 Definition
The PICO scheme is a tool for identifying key concepts in a topic and structuring literature searches. It is used for topics involving intervention or treatment.
The topic is structured according to four aspects:
| Aspect | Explanation | |
| P | Patient/Population | Description of the patient group or problem |
| I | Intervention | Description of the main intervention or treatment |
| C | Comparison | Alternative treatment or control group |
| O | Outcome | Desired output or target value |
1.2 Example
Case 1: Researching the technical literature
Your task is to research the relevant technical literature for a heat pack, for example to determine the state of the art.
The intended purpose of this device states:
“Heat pack for the treatment of lower back pain. The heat pack maintains a temperature of 40°C for 8 hours. It applies therapeutic heat, thereby relieving muscle tension and stimulating blood circulation in the tissue. Pain is reduced.”
You can take the following aspects from this intended purpose:
| P | Patients with back pain |
| I | Heat pack, therapeutic heat, heat application, heat therapy |
| C | — |
| O | Reduction of pain |
In this example, aspect C (Comparison) is not considered.
Case 2: Evaluation of a comparative therapy
Your question refers to a comparative therapy and is as follows:
“Is the use of a heat pack more effective than taking 1,200 mg of ibuprofen daily?”
This results in the complete PICO scheme. The aspects for the study on heat packs are as follows:
| P | Patients with back pain |
| I | Heat pack, therapeutic heat, heat application, heat therapy |
| C | 1200 mg ibuprofen, NSAID |
| O | Reduction of pain |
1.3 Variants
PICO can be expanded to include additional components depending on the issue and context.
1.3.1 PICOS
The additional component “S” stands for Study Design. PICOS includes the study design to narrow down the search, e.g., to Randomized Controlled Trials (RCTs), observational studies, or systematic reviews.
The PICOS aspects for an extended study on heat packs could be:
| P | Patients with back pain |
| I | Heat pack, therapeutic heat, heat application, heat therapy |
| C | 1200 mg ibuprofen, NSAID |
| O | Reduction of pain |
| S | Randomized Controlled Trials |
1.3.2 PICOC
The additional component “C” stands for Context Factors, e.g., patient comorbidities, environmental conditions, or settings that may influence the output.
The PICOC aspects for an extended study on heat packs could be as follows:
| P | Patients with back pain |
| I | Heat pack, therapeutic heat, heat application, heat therapy |
| C | 1200 mg ibuprofen, NSAID |
| O | Reduction of pain |
| C | Chronic pain, frequently seated, age > 75 years |
1.3.3 Further variants
In addition to PICOS and PICOC, some other extensions and variants have been developed for particular areas of application:
- PIPOH (Population, Intervention, Profession, Outcome, Healthcare Setting) for questions on the quality of care
- SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research Type) for qualitative studies in the field of nursing care
- PICO-D (Population, Issue, Context, Study Design) for psychological questions
The choice of the appropriate PICO variant depends on the specific research question and the information needs.
The more precise the question, the more targeted the search, and the less effort is required for the literature analysis.
2. Application areas of the PICO scheme
2.1 Literature research
People working in science, such as doctors, clinical affairs managers, medical writers, and pharmacologists, use the PICO scheme primarily to find clinical literature quickly and accurately.
These literature searches are mandatory for the approval of medicinal products and medical devices in the context of clinical evaluation:
- Determine the state of the art
- Carry out benefit-risk comparisons
- Carry out clinical evaluation (also with this information)
The PICO scheme is used to formulate search strings for the search masks of scientific databases. Therefore, PICO is used right at the beginning of the literature search, precisely when the strategy for the literature search is developed.
You can find more tips in the article on clinical literature research.
2.2 Evidence-based medicine
In evidence-based medicine, the PICO scheme helps to answer evidence-based questions such as:
- Is medicine A more effective than medicine B in treating condition X?
- Does minimally invasive surgery lead to better results than open surgery in patients with disease Y?
- Does vaccination Z reduce the risk of infection W in children compared to no vaccination?
- Is test A more accurate than test B in diagnosing disease V?
- Does a special rehabilitation program improve mobility in patients after a stroke compared to standard rehabilitation?
The PICO scheme is also used in evidence-based medicine to make recommendations or create medical guidelines.
2.3 Formulate an extended intended purpose
The PICO scheme is also helpful when formulating the extended intended purpose. For example, it can be used as a “checklist” to find out whether the intended purpose covers all aspects.
3. Evaluation of the PICO scheme
3.1 Advantages
The PICO scheme has proven its worth.
- It is widely used and creates a common mental model for collaboration.
- It facilitates the translation of clinical questions into search terms and thus helps to conduct faster, more structured, and precise literature searches.
- It helps to achieve better (especially more relevant) search results more quickly.
- It speeds up the evaluation because fewer irrelevant sources have to be read, evaluated, and excluded.
3.2 Regulatory obligation?
There is no explicit regulatory obligation to use the PICO scheme. However, it is considered best practice in evidence-based medicine and is recommended by many organizations.
When writing the clinical evaluation, manufacturers should consider relevant guidance documents such as MEDDEV 2.7/1 rev.4 and the MDCG guideline MDCG 2020-13, both of which suggest the PICO scheme. Therefore, many clinical evaluation investigators consider the PICO scheme mandatory and state of the art.
3.3 No alternatives(?)
Neither MEDDEV 2.7/1 rev.4 nor the MDCG mention an alternative to the PICO scheme for finding relevant keywords and search strings.
4. Creation of a PICO scheme
To ensure that you can successfully create and use the PICO scheme for your scientific research, here are some tips:
4.1 Match PICO scheme to task
The following categories and associations have been established
- Researching the state of the art
- Intended purpose (= intervention)
- Indications (patient population)
- Generic device group (= no comparison)
- Clinical benefit/performance/safety (outcome)
- Product search
- Name of own device
- Name of the company
- Search for benchmark devices
- Name of similar/equivalent device
- Name of the associated company
4.2 Database research in five steps
Once you have identified the specific keywords, you should carry out the following steps:
- Identify the question/intended purpose
- Identify synonyms per category
- (Translate these into English (In scientific databases, the language is English.))
- Link synonyms within a category with the Boolean operator OR and put them in brackets
- Link categories with AND
This creates a search string, which is used in search masks of databases such as PubMed, Embase, or Cochrane for literature searches.
Example: Search string for heat pack
| P | Patients with back pain |
| I | Heat pack, therapeutic heat, heat application, heat therapy |
| C | — |
| O | Reduction of pain, VAS score |
| Back pain AND (heat pack OR therapeutic heat OR heat application OR heat therapy) AND (pain reduction OR VAS score) | |
4.3 The most common mistakes
Typical mistakes when using the PICO schema and when searching the database are:
- The wrong Boolean operators are used.
- Brackets are set incorrectly.
- Unsuitable keywords are used, e.g., product specifications that are not described in detail in publications.
- Inconsistencies arise between PICO elements and the actual research question.
- PICO is used in the wrong context, e.g., in safety databases.
Too many hits in the literature search (> 1,000) or too few hits (< 5) can be an indicator that the PICO scheme has not been applied correctly.
4.4 Further tips
The following procedures have proven successful:
- Use precise and specific terms.
- Consider relevant synonyms for each PICO component.
- Adapt the schema to the specific question if necessary (e.g., PICOT or PICOS).
- Benefit from the “Advanced Search” in PubMed, which supports bracketing and Boolean operators.
- When searching for synonyms, use medical dictionaries, known publications in the field, and the PubMed mesh database.
This article on literature searches will help you when creating performance evaluations and clinical evaluations.
4.5 Benefit from support
The Johner Institute’s scientific and clinical experts will help you with all tasks related to clinical evaluation and post-market clinical follow-up, e.g., by:
- Reviewing your PICO scheme
- Reviewing your literature search
- Workshop on literature search; here, the use of the PICO scheme is explained and demonstrated in detail
- Creating the PICO scheme and carrying out the literature search
Get in touch, for example, via the contact page, if you need help or advice.
5. Conclusion
The PICO scheme is a proven and indispensable tool for formulating research questions and scientific research.
For medical device, IVD medical device, and pharmaceutical manufacturers, PICO supports all scientific and medical experts in particular.
The PICO scheme and its variants are considered state of the art.


