Both European and U.S. regulations place requirements on the labeling. However, the two legal systems do not understand the term entirely identically. Even the spelling differs: labeling in the USA, labelling in Europe.
In this article, you will learn what you need to keep in mind in each case when it comes to labeling. Speaking of spellings: We use American English in this article and save an “l” :-).
Labeling: What is it?
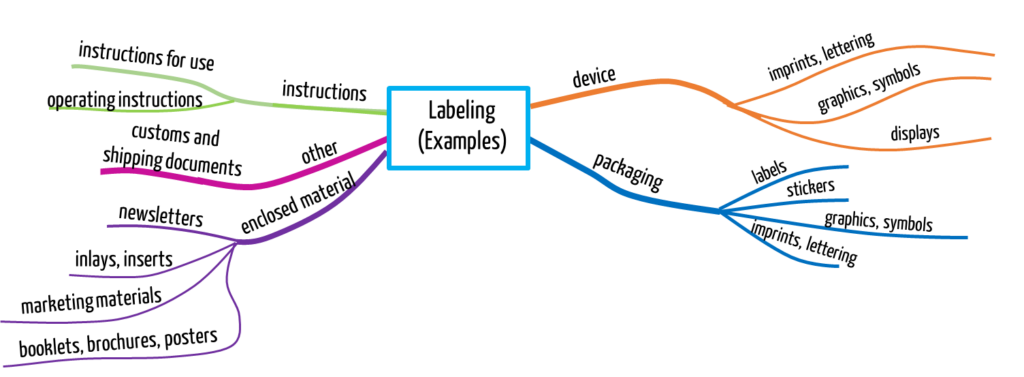
Fig. 1 gives an overview of artifacts that different regulations count as labeling.
a) Definition (FDA)
The FDA provides a definition:
„all labels and other written, printed, or graphic matter
- upon any article or any of its containers or wrappers, or
- accompanying such article“ at any time while a device is held for sale after shipment or delivery for shipment in interstate commerce.
Source: FDA 21 CFR part 201(m)
In this context, “labels” are, according to the FDA:
display of written, printed, or graphic matter upon the immediate container of any article […].
Source: FDA 21 CFR part 201(k)
The FDA provides the following as examples of labeling:
- operating instructions or instructions for use
- printed matter on packaging (e.g., graphics, symbols, text)
- labels
- flyers, booklets, brochures, posters
- inserts, inserts
- circulars
I.e., “accompanying” marketing material also counts as labeling. The FDA wants the term “accompanying” to be understood literally. Even customs and shipping documents can also be included in the definition.
b) Definition (ISO 13485)
The definition of ISO 13485 has changed slightly over time. In the 2012 version, it still stated:
Any written, printed, or graphic information
- on a medical device or any of its containers or other packaging or
- as an enclosure with the medical device,
that relates to the identification, technical description, and medical device use, excluding shipping documents.
Source: ISO 13485:2012
Thus, the translation of the term “labeling” was “marking.” Unlike the FDA, shipping documents are not covered by the term.
The latest version of the standard, ISO 13485:2016, states:
Label, instructions for use, and any other information that is related to identification, technical description, intended purpose, and proper use of the medical device, excluding shipping documents.
Source: ISO 13485:2016 to GHTF/SG1/N70:2011, Clause 4.
This definition uses the term “label”. The standard does not define this term.
c) Medical Device Regulation
The Medical Device Regulation (MDR) uses the term “labeling” without defining it. The following passages suggest that the MDR considers labeling as the inscriptions directly on the device:
- “In the labeling, instructions for use, making available, putting into service and advertising of devices, it shall be, […]”
- “the instructions for use and the labeling.”
The MDR distinguishes instructions for use and marketing materials on the one hand and “labeling” on the other.
d) Other regulations
In this context, IEC 60601-1 uses the terms “identification,” “marking,” and “documents.”
e) Conclusion
Different regulations’ definitions of labeling/marking are comparable but not identical. Therefore, ensure that you, your colleagues, your customers, and your notified body are discussing the same thing.
Regulatory requirements for labeling
Overview
The MDR assumes that the labeling on the product itself (labeling) and the instructions for use exist and contain the information set out in Annex I, Article 23. Other regulations set more specific requirements:
- EU Regulation 2226/202 regulates the use of electronic instructions for use.
- In its informative annex, ISO 24971 recommends considering the labeling in the risk analysis.
- ISO 13485:2016 requires that “labeling” be part of the medical device file. During production, monitoring that labeling and packaging are compliant is necessary.
- IEC 60601-1 and standards referenced therein, such as DIN EN 60445 and DIN EN 60447, provide numerous rules, which you can read about below.
- Further particular standards of the IEC 60601 family supplement these specifications.
- ISO 15223-1 regulates the symbols that manufacturers of medical devices must use.
- ISO 20417 expands and presents the labeling requirements of the MDR and IVDR.
Examples of IEC 60601 requirements
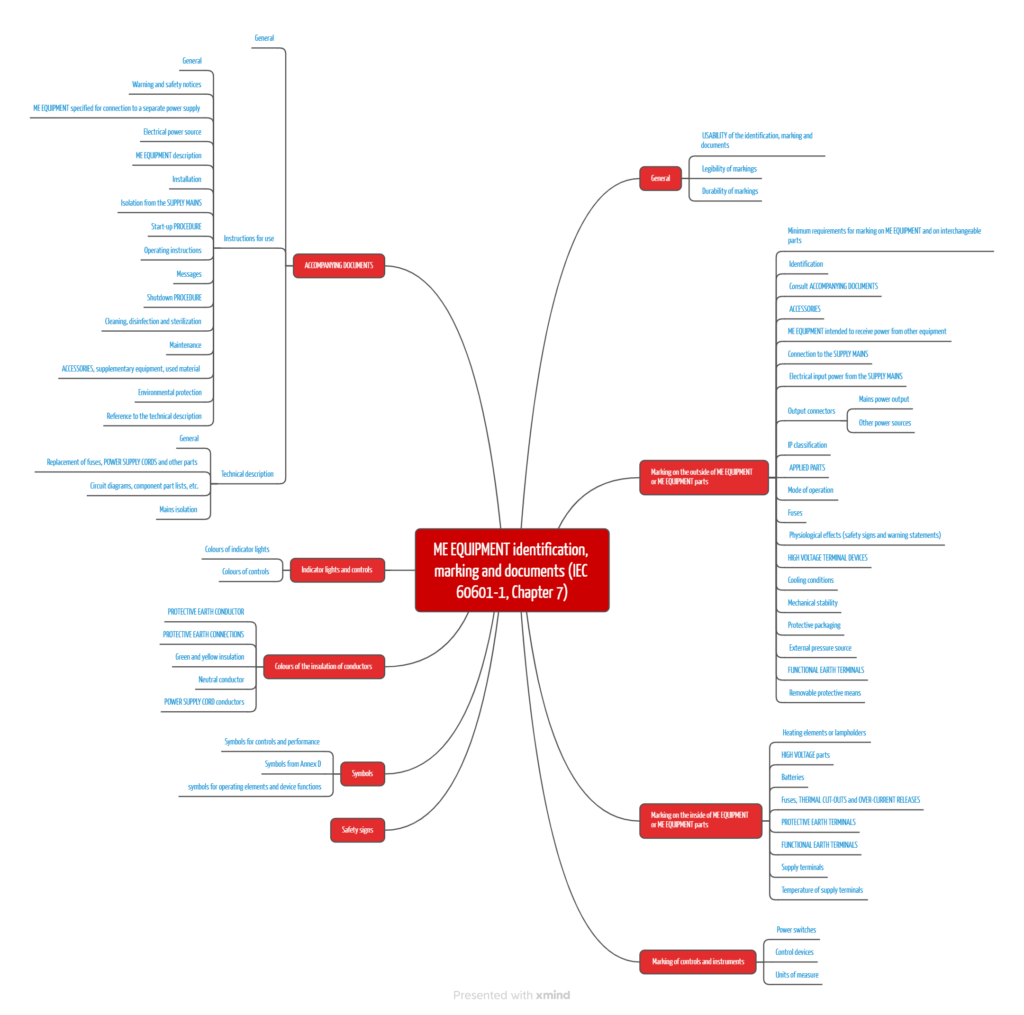
IEC 60601-1 devotes an entire chapter to the topic of labeling. It deals with using symbols and colors, required inscriptions, instructions for use, permanent legibility, etc.
(click to enlarge)
The most important requirements thus relate to:
- inscriptions on the outside of the device
- markings on the inside of the device
- labels on operating elements and displays
- instructions for use
- technical description
- other aspects, such as colors, symbols and signal lamps
Summary
Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Therefore, manufacturers must understand which risks are minimized and which risks are created. At the same time, they must comply with numerous regulations.


