From 2017-2022 the FDA offered a “Precertification (Pre-Cert) Pilot Program” to specific companies for standalone software to simplify and accelerate the approval process for digital health devices.
The authority provides detailed information on its website.
This article summarizes the most important aspects and provides a quick overview.
Precertification (Pre-Cert) Pilot Program
The FDA has recognized that software
- is and must be changed very quickly,
- is playing an increasingly important role in healthcare, and
- the number of applications for approval (especially 510(k)) will rise sharply.
The new approach
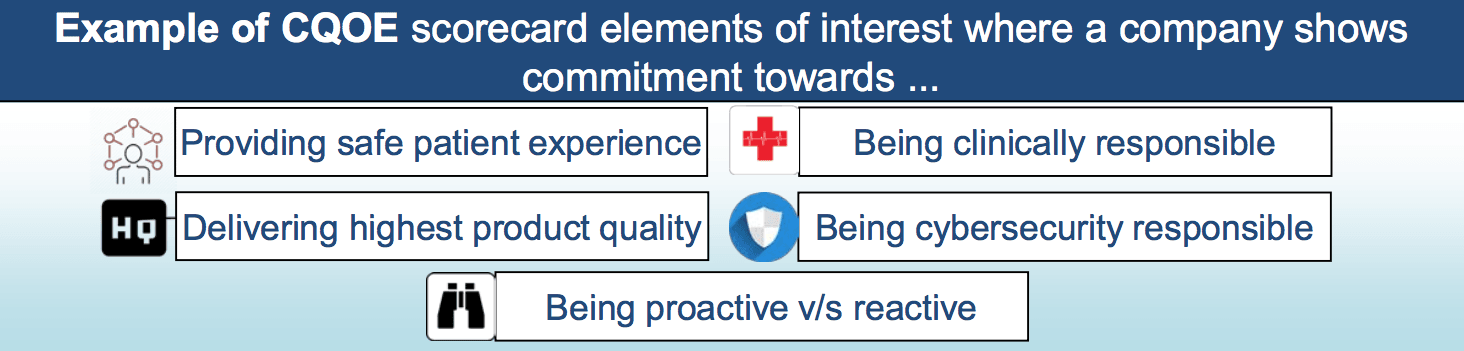
The agency is, therefore, experimenting with a new approach that almost deserves the attribute “revolutionary.” The idea behind it is that it is no longer primarily the device that decides whether it is eligible for approval but the organization. It must prove that it is capable of
- ensuring that patients can handle the devices safely,
- acting in a clinically responsible manner,
- providing devices of the highest quality,
- fulfilling its responsibility regarding cybersecurity, and
- acting proactively and not (only) reactively.
The FDA wants to measure the organization’s capability to live this culture using key performance indicators.
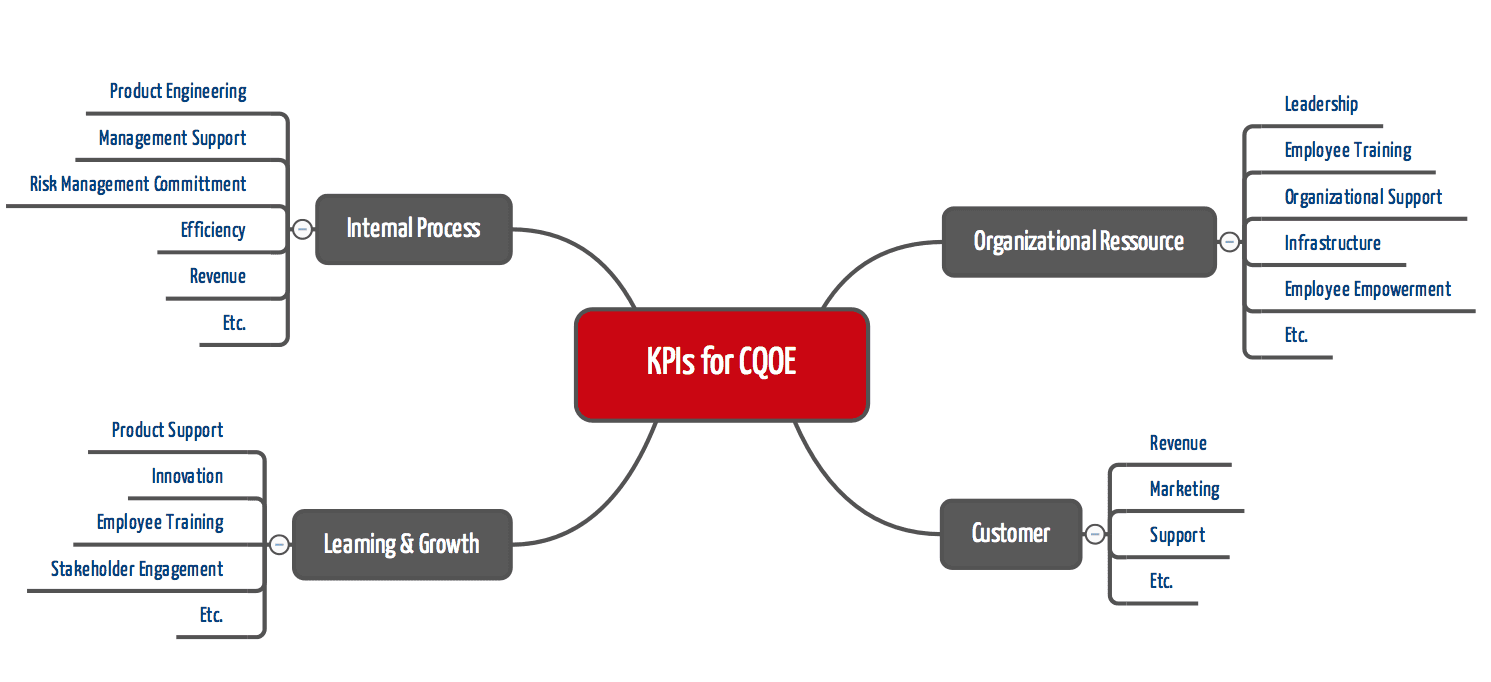
In doing so, the FDA is shifting the focus from
- product characteristics to the capabilities of an organization (quality management system),
- compliance monitoring by the authority to compliance monitoring by the company itself,
- pre-market to post-market activities.
In particular, risk management, employee training, post-market monitoring, including post-market clinical follow-up, and usability engineering will likely become increasingly important.
In the record of a webinar “Digital Health Software Precertification (PreCert) Pilot Program,” the FDA presents its new approach:
The slides for this webinar can be found here.
The FDA describes the Software Precertification Program in a 17-page document.
Facilitations due to the FDA Precertification Program
- The 21st Century Cure Act has already brought relief by excluding many software products from the definition of a medical device.
- Further relief is to be provided by accelerating the premarket review process.
- Software devices with a low risk may even be marketed without such a review – if the organizational requirements are met.
Participation in the Pre-Cert Pilot Program
The FDA wants to try out the program as part of a pilot. The number of participants is minimal. Prominent names are involved with Apple, Johnson & Johnson, and ROCHE.
The program only applies to “Software as a Medical Device” if the software does not control any other devices. However, it is permitted to receive data from such devices.
Results of the pilot program
In 2022, the FDA published a report on its experience with the Pre-Cert Program. It openly admits that the experiment was (methodologically) difficult, partly due to the regulatory framework. Nevertheless, the FDA can deduce from its experience that new approval procedures are helpful for modern technologies but that these require legislative changes.
Even if the experiment was not an unqualified success, the FDA shows us how to drive regulation forward: Through a scientific approach (regulatory science) that also uses experimentation as a method.
The work is, therefore, an essential contribution to evidence-based regulation.
Conclusion
The FDA certainly won’t become reckless. However, it recognizes that traditional mechanisms for market approval of software are unsuitable because they are too heavyweight and/or ineffective. The balance between patient safety and the timely availability of devices was no longer sufficiently given.
The fact that the authority is talking about the trust it wants to place in companies is both unusual and welcome.
It will be interesting to see whether the European authorities offer a comparable “Precertification Program.” Rule 11 of the MDR suggests the opposite.
Would you like to market your device in the USA? Professor Johner and his team will help you to overcome the regulatory hurdles quickly and easily.
Change history
- 2022-09-30: Output of the FDA from 2022 added


