The Global Medical Devices Nomenclature (GMDN), the Universal Medical Device Nomenclature System (UMDNS), and the Classificazione Nazionale dei Dispositivi Medici (CND) are nomenclatures for medical devices. The latter serves the EU as the basis for the European Medical Device Nomenclature (EMDN). Finally, there are the MDA/MDN codes in accordance with the EU Implementing Regulation (EU) 2017/2185.
This article explains when you need the GMDN or UMDNS and when you need to use which classification system.
1. Areas of application of the EMDN, GMDN, UMDNS, CND and MDA codes
a) Registration of devices with DIMDI
Section 25 of the Medical Device Regulation requires that the “person responsible [… who] places medical devices […] on the market for the first time must notify the competent authority, stating the address, before commencing the activity”. The corresponding regulation would regulate the details. This is the DIMDI regulation.
Section 2 of the DIMDI regulation states: “A nomenclature specified by the German Institute for Medical Documentation and Information using the central registration system shall be used for the designation of medical devices.” These are currently the UMDNS codes.
However, as the DIMDI writes on its website, the “UMDNS and EDMS are to be replaced in future by the Global Medical Device Nomenclature (GMDN). The EU Commission intends to translate the GMDN into the languages of the member states to ensure a uniform designation of medical devices”.
As long as the EUDAMED is not sufficiently functional, there is an obligation to notify placing on the market via the DMIDS (German Medical Device Information and Database System).
b) Reporting incidents to the BfArM
Formerly, according to the MPG and MPSV, today, according to the MPAIMV, manufacturers are obliged to report incidents and occurrences to the BfArM.
Anyone who operates or uses devices professionally or commercially must immediately report any suspected severe incidents to the competent higher federal authority. Sentence 1 shall apply mutatis mutandis to doctors and dentists who become aware of alleged severe incidents during their professional activities.
MPAIMV § 3 (German)
This higher federal authority requires the notifications to be recorded in its online system:
(1) The notifications under Section 3 shall be made for central recording via the German Medical Devices Information and Database System pursuant to Section 86 of the Medical Devices Implementation Act. The notifications pursuant to § 4 sentence 2 may be made for central recording via the German Medical Devices Information and Database System pursuant to § 86 of the Medical Devices Implementation Act.
MPAIMV § 6 (German)
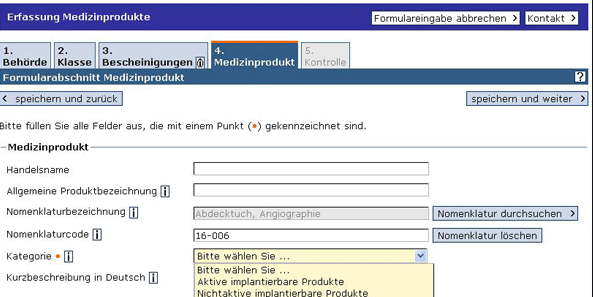
A code according to a nomenclature is expected:
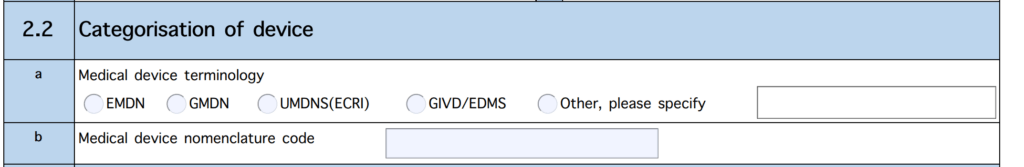
Manufacturers can choose between EMDN, GMDN, and UMDNS, as well as EDMS (for IVDs) as nomenclatures.
c) Notifications and registration in EUDAMED
The MDR also requires the use of nomenclatures in the EUDAMED. These must even be made available free of charge, as Article 26 states:
“To facilitate the functioning of the European database on medical devices (‘Eudamed’) as referred to in Article 33, the Commission shall ensure that an internationally recognised medical devices nomenclature is available free of charge to manufacturers and other natural or legal persons required by this Regulation to use that nomenclature”.
Manufacturers must enter this code when registering devices with the UDI-DI and other information about the device, such as risk class and trade name.
The EU has decided to use the Italian CND codes as the basis for the “European Medical Device Nomenclature” (EMDN). These are then the codes to be used when registering devices in Eudamed.
Read more about Unique Device Identification (UDI) and the EU Medical Device Regulation MDR here.
d) Auditing: number of technical documentations to be audited
The MDR uses the terms “generic device group” and “category of devices.” This implies a hierarchy of device types, which the GMDN also takes up. Specifically, the MDR fixes the amount of technical documentation to be checked during the audit:
- Manufacturers of class IIb devices […] shall be subject to a conformity assessment […] including an assessment of the technical documentation […] of at least one representative device per generic device group.
- Manufacturers of class IIa devices […] shall be subject to a conformity assessment […] including an assessment of the technical documentation […] of at least one representative device for each category of devices.
This presupposes that medical devices can be clearly divided into “Generic Device Groups” and “Categories of Devices.” The MDR does not even define the terms. More on this below.
e) Other areas of application
The article on generic device groups and device categories mentions other areas of application, such as the Periodic Safety Update Reports (PSUR), which manufacturers “only” have to prepare per device group or device category in accordance with Article 86(1).
2. The EMDN codes
The European Nomenclature on Medical Devices (EMDN) has prevailed for registering devices in the EUDAMED. It is based on the Italian system of CND codes. CND stands for Classificazione Nazionale dei Dispositivi medici, meaning National Classification of Medical Devices. This was determined by the Medical Device Coordination Group MDCG 2019-13 guidance document.
a) The history and the Italian version
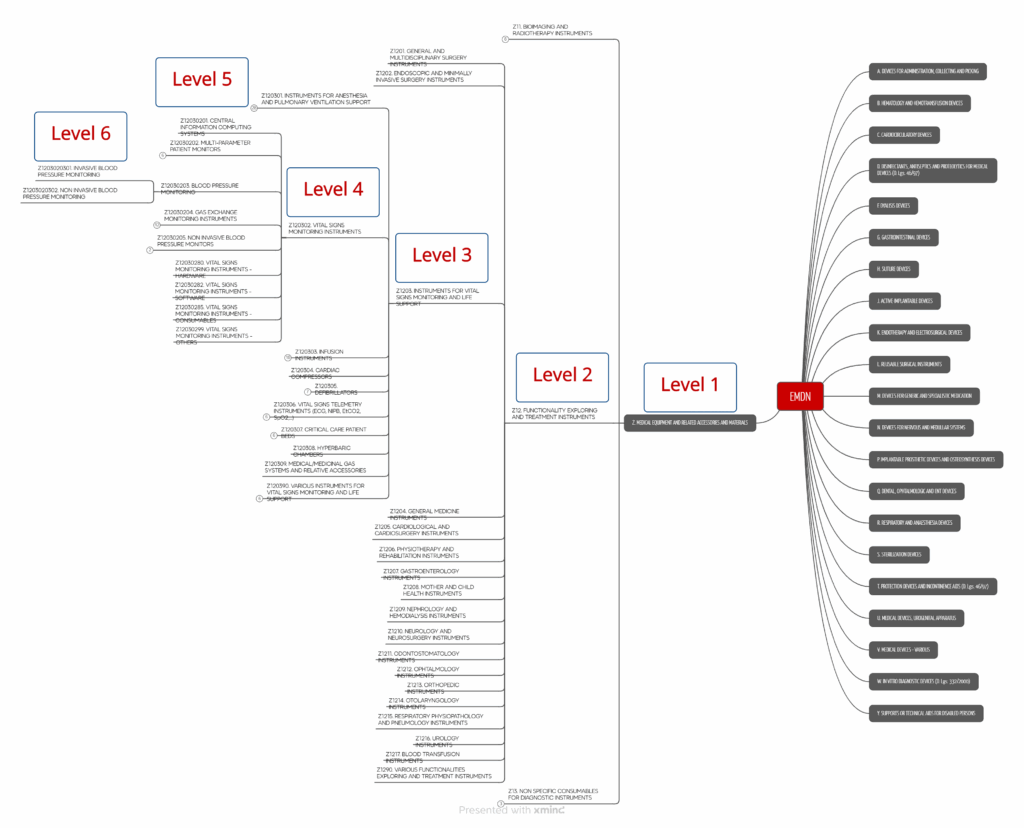
The European Commission has published a guidance document that provides background information on the Italian nomenclature CND. The nomenclature is also publicly available on the Italian Ministry of Health website. It divides medical devices into up to seven levels.
Therefore, a translation program is helpful as the page, and the terms are only available in Italian. However, the Ministry provides the translation of the CDN codes as a PDF and as an Excel file.
At 355 pages, this document is very extensive. How completely and granularly this ontology classifies medical devices remains to be seen. The document recognizes at least over 150 devices that contain the term “software.”
The taxonomy recognizes almost 7,000 categories, of which
- 22 codes at the first level,
- 146 codes at the second level,
- 724 codes at the third level,
- 1,681 codes at the fourth level.
b) The current list of EMDN codes
The EU published a new version of the EMDN codes in the fourth quarter of 2021. In addition to editorial changes, there are new codes under the digits J, W, and Z. This affects active implants, IVDs, and accessories, including software.
The website contains a link to download this taxonomy and a navigable tree structure that clearly displays this taxonomy and allows you to search for keywords.

The MDCG has defined the fourth-level codes as the generic device groups.
Read more about generic device groups and product categories here.
However, this taxonomy’s fifth and sixth levels are not the level at which the base UDI-DI would be assigned. A typical basic UDI-DI may only include devices with identical “essential design and manufacturing characteristics.” However, these are not classification characteristics in the EMDN, neither on the fifth nor the sixth level.
c) Further development of the EMDN code
In the guideline (MDCG 2024-2), the MDCG describes the process by which the EMDN codes are to be updated. This is to take place annually in the future. However, it does not explain the logic by which the expert groups involved will decide in which cases these codes will be adapted and how.
The EU has introduced an EMDN Helpdesk. The website also contains FAQs, MDCG publications, and further information.
3. The UMDNS codes
a) Structure of the UMDNS code system
The UMDNS codes can be downloaded directly from the DIMDI website. It distinguishes between more than 5,000 terms, although these are not organized hierarchically. The codes do not follow any recognizable system.
b) Examples with reference to the software
The UMDNS does not recognize the term “software,” but it does assign codes for “information systems” (code 17-222) or “information system, cardiology” (code 18-118).
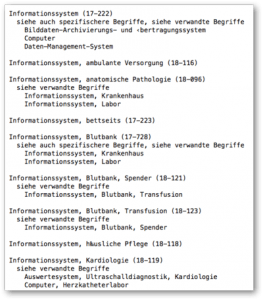
c) Conclusion and criticism of UMDNS
The UMDN system is completely outdated. However, it is available free of charge. The lack of hierarchy (UMDNS is not a classification but a taxonomy) makes searching and systematic evaluation difficult. The fact that the DIMDI works with UMDNS codes and the BfArM prefers GMDN codes says something about the coding systems and the coordination between the authorities.
4. The GMDN codes
a) Costs
In contrast to UMDNS, the GMDN codes were not available free of charge. The GMDN Agency charged an annual fee of up to 4,000 euros, depending on annual turnover. Now, the codes are available to all target groups free of charge.
The GMDN codes are well-established internationally. The European Commission is therefore mapping the CND nomenclature to the Global Medical Device Nomenclature (GMDN) codes to meet the requirements of the Medical Device Directive (MDR) and the In Vitro Medical Devices Directive (IVDR).
“The GMDN Agency welcomes the recent announcement from the European Commission regarding its intention that the nomenclature used in the EU for naming and categorizing medical devices will, in future, need to strongly align to the GMDN (https://ec.europa.eu/docsroom/documents/34264?locale=en).”
GMDN Agency
Thanks to B. Reszel, J. Schmidt and Dr. K. Martins!
b) Structure 1: Overview of the GMDN code system
The GMDN system has a hierarchical structure. It represents a taxonomy, as seen here using the example of computers/software.
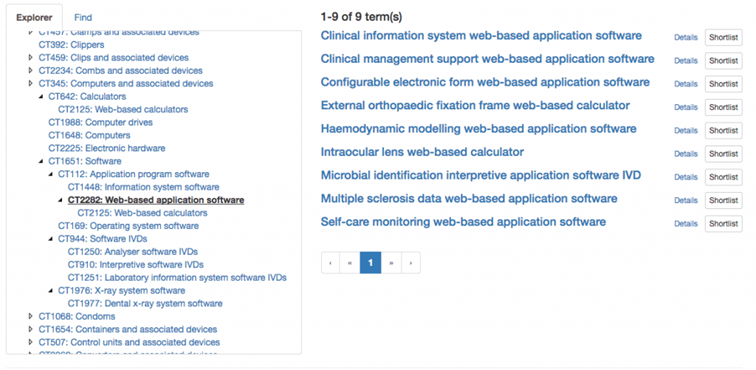
Strictly speaking, there are actually two hierarchies: one by name and one by use case.
Each term has the following attributes:
- Name
- Description
- Code
- Status (e.g., “Active”)
- Creation date
- Date of last modification
Some of the terms are translated. The translation concerns the name and the description. The translations also have a translation date.
c) Examples
The term “Clinical information system web-based application software” is described as follows:
“An application software program designed as an Internet-based information system to support the administrative, clinical, and quality control activities associated with the provision/utilization of healthcare within a specific clinical specialty (e.g., orthopaedics, general surgery, neurology, ophthalmology, oncology, rheumatology, dermatology). It can be available on any web-enabled interface (including mobile devices at point-of-care) and is intended for use by healthcare professionals in a clinical setting.”
d) Structure 2: Polyhierarchical and polyaxial system
The term is located within the first hierarchy (by name):
- Computers and associated devices > Software Application program software > Web-based application software
Interestingly, this is not a monohierarchical and monoaxial hierarchy. Consequently, there are further hierarchy paths:
- Computers and associated devices > Software Application program software > Information system software
- Information systems and associated devices > Information system software

The term “information system software” can be found both under the main term “information systems and associated devices” and under the main term “computers and associated devices.”
The classification of the term in the “by use” hierarchy is not entirely comprehensible:
- Orthopedic devices > Implantable joint prostheses and associated devices
The GMDN structure consists of three levels:
- device category (e.g., “Computers and associated devices”)
- generic device group (e.g., “Software Application program software”)
- device type (e.g., “Web-based application software”)
As mentioned above, these three levels are crucial for auditing or reviewing the technical documentation.
e) The codes
The codes do not allow any conclusions to be drawn about the hierarchy. For example, the term “Information systems and associated devices” has the code CT223, and the sub-term “Information system software” has the code “CT1448”.
The specific characteristics – in our example above, “Web-based application software” – i.e., the “leaves” in this hierarchy, do not have their own code.
5. The MDA/MDN codes
a) General
The EU Implementing Regulation (EU) 2017/2185 defines the product categories. The EU system is also structured hierarchically.
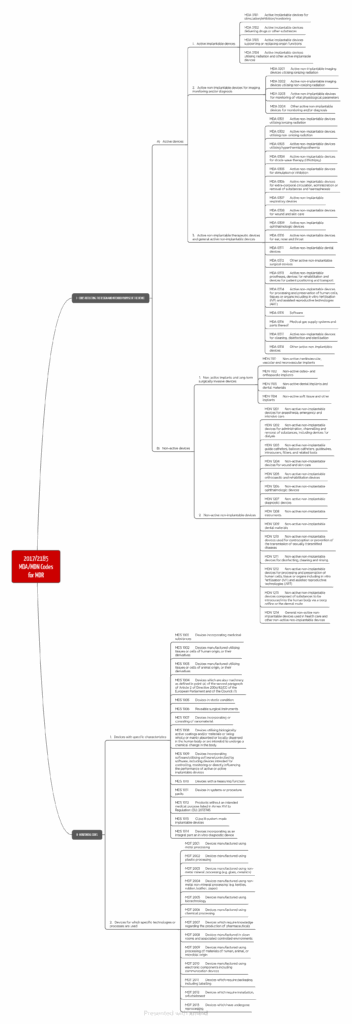
The EU distinguishes between 71 product categories for devices covered by the MDR and 79 product groups for IVD products.
This article explains the significance of the product categories for the MDR and IVDR.
6. The Chinese code system
The Chinese company Zhixie provides a search mask that can be used to search for the corresponding codes. This is particularly useful because it allows the class of the devices to be determined quickly.
7. Conclusion
The GMDN coding system was preferred by the EU Commission and commissioned based on ISO 15225. The commercially operating GMDN Agency actively manages these terms. They are better maintained than those of the UMDN system. The publicly available Italian CND system has a good chance of being used as the standard for the EUDAMED. A mapping of both is planned.
The coding systems are required for the following purposes:
- Registering devices (currently with DIMDI via UMDNS)
- Report incidents (preferably via GMDN)
- Enter devices in the EUDAMED database (probably via GMDN in the future)
- Deciding how much technical documentation needs to be checked in the audit in accordance with MDR
It is to be hoped that the coding systems
- are available free of charge,
- are consolidated (i.e., only one and no longer three or more are used in parallel),
- enable a convenient search,
- are/remain stringently structured and
- are continuously maintained (including competent and rapid support).
Would you like support in registering your device, reporting an incident, or meeting other regulatory requirements? Professor Johner and his team are happy to help!
Get in touch
Change history
- 2024-02-08: Chapter on EMDN rewritten and moved up due to greater importance. Regulatory references in chapter 2 updated.
- 2021-11-22: Section 4. b) added (New version of EMDN codes, reference to EU website)



Hi, could you please get in touch with the GMDN Agency? We have noticed that some of the information you provided regarding GMDN is incorrect and we would like to have a conversation to resolve the issues.
Dear Deniz,
I’m happy to fix any mistakes. My I contact you via the provided email address?
Christian
Hi, yes, please contact me at the email address provided. Thanks.