Cytotoxicity testing can be used to test a sample to determine how much it can damage, or even cause the death of, human cells. Standards and legislation require manufacturers to demonstrate that their devices are not cytotoxic.
With a good test setup, it is possible to detect whether a sample is 100% cytotoxic, i.e., highly cytotoxic, or 0% cytotoxic, i.e., not cytotoxic. In contrast, an unsuitable test set-up can lead to the cytotoxicity not being determined correctly.
And this can lead to the non-detection of cytotoxic substances that may have irritating, sensitizing or, in the worst case, even carcinogenic/mutagenic/reprotoxic (CMR) properties. With fatal consequences for patients, users and third parties.
Therefore, carefully designing the tests is vital. This article explains how to do this and gives you 7 power tips that will help you select a laboratory.
1. Definitions and limits
ISO 10993-1 defines cytotoxicity as follows:
„Cytotoxicity evaluation is an initial screen for the presence of potential adverse biological effects from the medical device. Therefore, all medical devices having direct contact or indirect contact with the body, irrespective of contact duration, shall be evaluated for potential for cytotoxicity.“
Source: ISO 10993-1, 6.5.2
EN ISO 10993-5 also defines the limits and, therefore, when a device/material/extract has cytotoxic effects. The standard gives two criteria for determining whether a substance should be classified as cytotoxic:
“Reduction of cell viability by more than 30% is considered a cytotoxic effect.”
“The achievement of a numerical grade greater than 2, based on Tables 1 and 2, is considered a cytotoxic effect.”
Source: EN ISO 10993-5, Section 8.5
2. Quantitative and qualitative evaluation
That there are two approaches to determining whether something is cytotoxic is logical given the two ways to analyzing cytotoxicity tests: quantitative evaluation and qualitative evaluation.
| Evaluation | Qualitative evaluation | Quantitative evaluation |
| What | Cell changes, growth inhibition | Cell death, inhibition of cell growth, cell proliferation, colony formation |
| With | Microscope | Assay/staining, etc. (MTT, XTT, BCA, etc.) |
| How | Visual assessment | Amount of protein, release of enzymes, reduction of vital dye… |
| Presentation of results | Grade (0-4) | Percentage (0-100%) |
| Limit value | > Grade 2 | > 30% |
While the quantitative evaluation usually relies on assays and evaluation by photometer, the qualitative evaluation is purely visual using a microscope. In a qualitative microscopic evaluation, the cells are assessed visually and the sample is graded.
| Grade | Reactivity | State of the culture |
| 0 | None | … no cell lysis, no reduction of cell growth |
| 1 | Slight | <= 20% of cells are round, loosely attached… only slight growth inhibition observable |
| 2 | Mild | <= 50% of the cells are round, …no extensive cell lysis, <= 50% growth inhibition observable |
| 3 | Moderate | <= 70% of the cell layers contain rounded cells or are lysed… > 50% growth inhibition observable |
| 4 | Severe | Nearly complete or complete destruction of the cell layers |
Under the microscope, the sample might look like this:
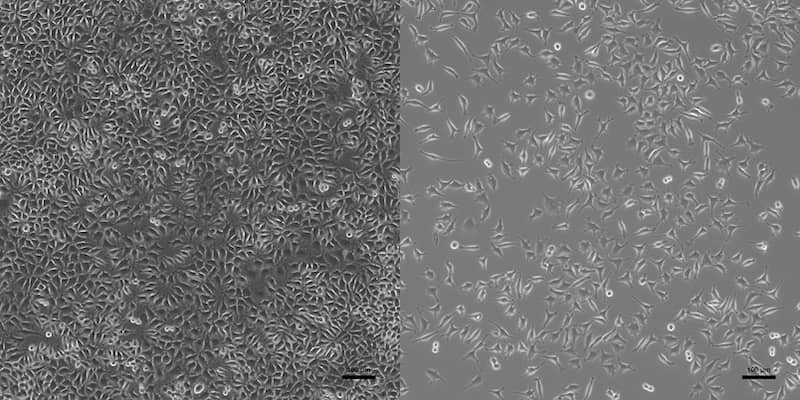
The cytotoxicity test is not a hard pass or fail test. Even cytotoxic results can be acceptable for certain materials and applications.
3. Regulatory requirements
Regulations like the MDR require proof of the biocompatibility of all materials that come into direct or indirect contact with patients.
The cytotoxicity test is THE standard test for biocompatibility according to ISO 10993-1.
The article on biocompatibility and ISO 10993-1 will give you an overview of the topic and introduce you to additional regulatory requirements.
Cytotoxicity testing is essential for every medical device category, contact duration and type of contact. In addition, it also used to test final cleaning as part of the validation (ISO 19227). It has been a standard test for batch releases for years.

This is due to the cytotoxicity test’s strong sensitivity, which makes it ideal as a screening test.
A cytotoxicity test is a screening test that can detect a wide range of production tools, monomers, metal ions, plasticizers, cleaning agents, and disinfectants, etc. But not all in toxicologically relevant concentrations!
However, the cytotoxicity results themselves do not allow conclusions to be drawn about the cause of the effect. In addition, a passed cytotoxicity test is not a guarantee of the absence of critical residues or that problematic substances are not released by the device/material. The reason for this is that the test is a very sensitive screening test overall (i.e., it detects a lot of different substances well even at low concentrations) but is limited and no longer works if the concentration is too low, even though it may still be highly toxicologically relevant.
Cytotoxicity testing alone is not sufficient to demonstrate biocompatibility or the purity of medical devices. Additional analyses adapted to the device, its use and the question should always be performed.
4. Cytotoxicity testing process
A cytotoxicity test essentially consists of 3 steps.
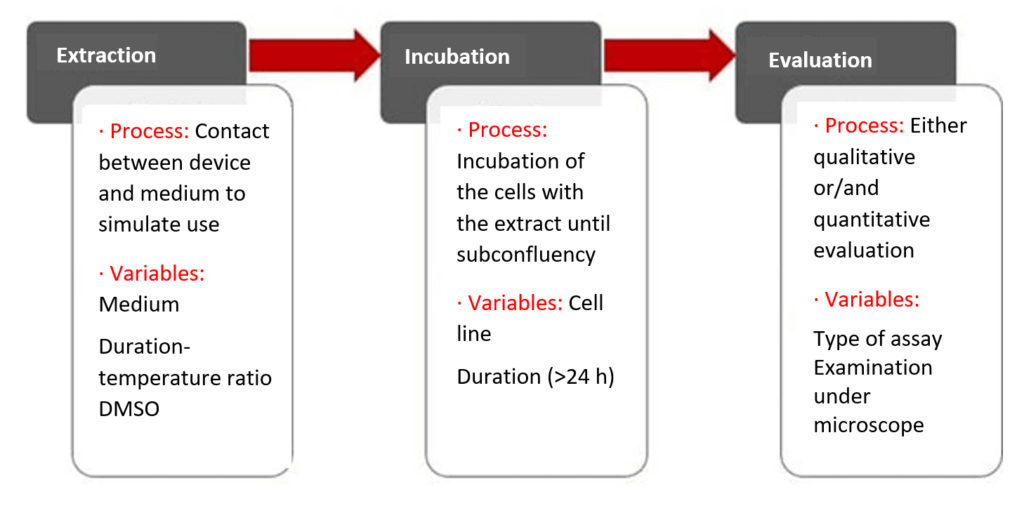
Step 1: Extraction
The first step is the extraction. For this, the device is brought into contact with an extraction medium and extracted in an inert vessel over a specified period of time, at a specified temperature and with an extraction ratio adapted to the device. Details can be found in EN ISO 10993-12.
The extraction medium can vary widely but is usually a cell culture medium with serum added, sometimes also with DMSO (dimethyl sulfoxide) added as a migration enhancer.
Step 2: Incubation
After extraction, the extract is incubated together with the cells. There are several ways of doing this: the cells can be pre-incubated (pre-grown) with no extract or be grown together with the cells.
The duration of incubation is variable: it is never less than 24 h and can last up to several days. The cell line L929 (mouse fibroblasts) is the established standard for the test. In principle, other cell lines can also be used.
The cells then incubate (grow) in the presence of the extract until subconfluency (confluency = cell density) is reached.
“approximately 80% confluency, i.e., the end of the logarithmic phase of growth”
Source: EN ISO 10993-5, 3.6
Step 3: Evaluation
After the incubation to a subconfluent (80% confluency) cell lawn, the influence of the extract on cytotoxicity is evaluated. This is done either qualitatively or quantitatively, as described above.
You must now be wondering what influence all the variables can have on the test and, therefore, on your test result.
The answer is: you can never get a bad test result from a very good device. A device that does not release or contain on its surface cytotoxic substances will never become cytotoxic as a result of the test parameters being changed.
But you can lower the sensitivity of the test by using certain combinations of test parameters so that the cytotoxicity of a bad device is reduced from 100% to 0%. We have seen numerous examples of this in the last 10 years.
Specify all test parameters so that test results are fully comparable in the event of retesting and avoid commissioning too many test laboratories for one device. If you have approved several laboratories as suppliers, make sure that the test parameters are as comparable as possible.
Do not mask poor results with tests designed to have low sensitivities. Cytotoxic results always have a very serious cause and should be looked into as a matter of urgency. Otherwise, the results will eventually catch up with you. In the case of product contamination, in particular, it is not uncommon for this to increase over time if the cause is not eliminated.
The testing of extracts described above is by far the most common test setup. For the manufacturer, knowledge of the above interrelationships is enough.
Please feel free to contact the Johner Institute’s experts (e.g., by email) if you would like to know more about the procedure and the performance of other special test variants (testing by direct contact, agar diffusion and filter diffusion).
5. The ISO 10993-5 standard
The title of EN ISO 10993-5 is “Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity.” At 46 pages long, the standard is relatively manageable. Much of the standard (Annexes A-D) is devoted extensively to how to implement specific test setups and is primarily of interest for laboratories or for troubleshooting.
The sections relevant to the normal manufacturer finish on page 21.
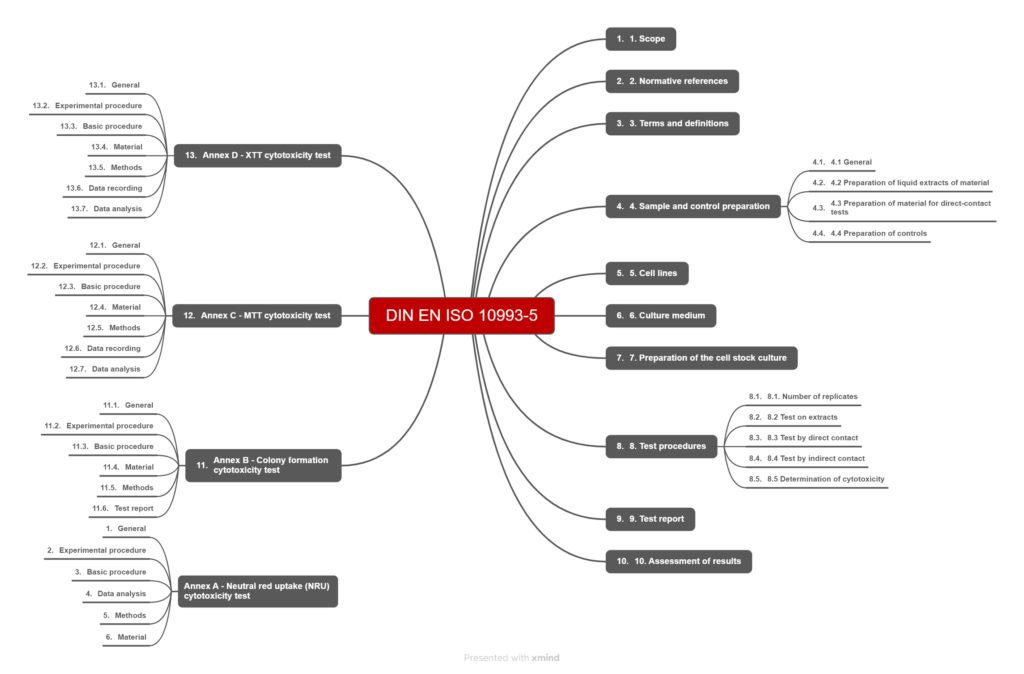
In addition to the requirements of EN ISO 10993-5, manufacturers must also take into account the requirements of ISO 10993-1 and EN ISO 10993-12 for the tests.
6. Interlaboratory comparison for cytotoxicity (ISO 10993-5)
The statements made in this article have also been confirmed by an interlaboratory comparison conducted by the Johner Institute in 2021.
Background
The Johner Institute organized a first-time independent interlaboratory comparison for cytotoxicity testing according to ISO 10993-5 from the perspective of a medical device manufacturer. Until then, laboratories themselves had to seek comparative testing with other laboratories. We were convinced that an interlaboratory comparison in the cytotoxicity test is possible, even if the test, with its broad normative specifications and interpretations, is considered too differentiated to be used for comparison.
The interlaboratory comparison was extended from the national to the international level; 52 laboratories participated out of more than 250 invited laboratories. The organization of the interlaboratory comparison was a pro bono performance of the Johner Institute to create the incentive for the highest possible number of participants and thus generate added value for all involved.
Implementation
Each laboratory received 2 different materials in the form of sterile tube pieces. One cytotoxicity test per material was to be performed. The materials used were not known to the laboratories. Apart from the extraction specifications, no other test parameters were specified by the Johner Institute.
Polyvinyl chloride (PVC with DEHP) and polyethylene (PE) were selected because both are typical materials for medical devices, and the cytotoxic potential was generally known.
High-quality polyethylenes are generally considered non-cytotoxic, whereas PVC materials are expected to have cytotoxic potential (inhibition of proliferation > 30%), depending on the formulation.
The assumption of cytotoxicity of both materials was also confirmed again by a repeated measurement at a laboratory in advance.
The anonymous participation was particularly important to us. All data were treated with special sensitivity. The objective was that no test laboratory could be clearly identified from the outputs or test parameters.
Results
The results were alarming. For the cytotoxic PVC, which was assumed to be cytotoxic undoubtedly, results of 0 % – 100 % proliferation inhibition were obtained.
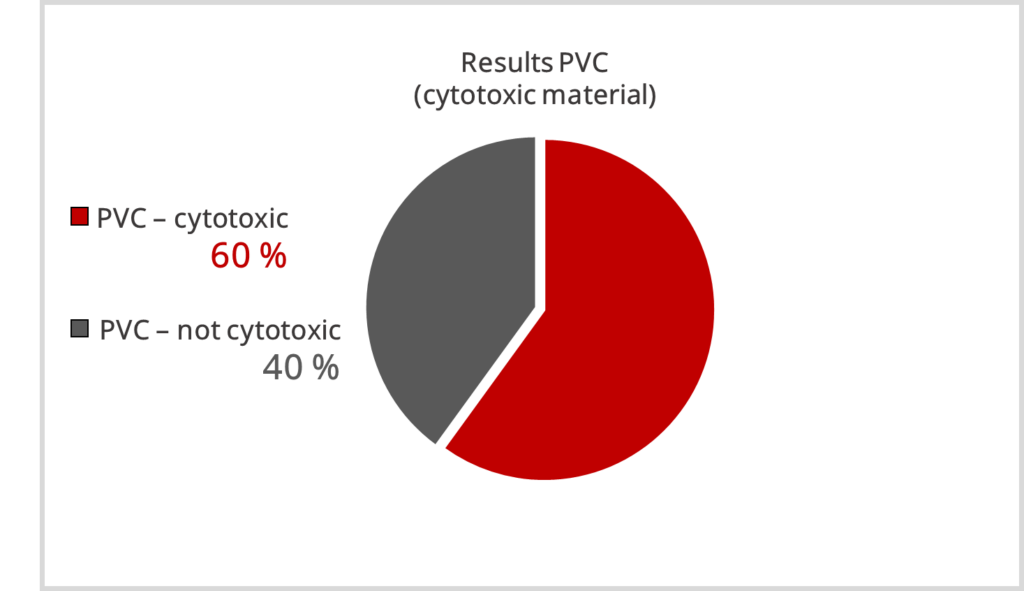
For the non-critical material PE, 100% clear results were assumed. Unfortunately, this was not the case; obvious cytotoxic results were also obtain here, with most results below 30 % proliferation inhibition.
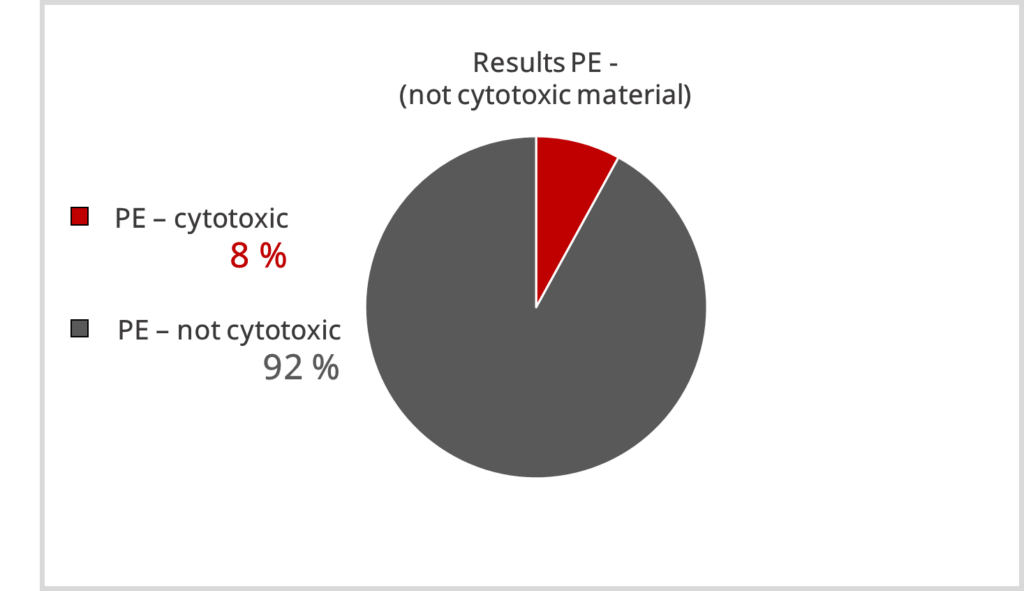
In summary, materials that are not classified as cytotoxic are identified as such in most cases. However, materials that are assumed to be cytotoxic are not identified as such by all laboratories.
Result: Laboratories do not provide comparable results for an identical material or medical device
For details see here our peer-reviewed paper “Toxic or not toxic? The specifications of the standard ISO 10993-5 are not explicit enough to yield comparable results in the cytotoxicity assessment of an identical medical device”
The comparison of the results per laboratory confirms the very different sensitivities of the test designs. All sensitivities and all conceivable gradations are present. The numerous variable parameters can obviously have a huge influence on the sensitivity of the test system.
Result: The specifications of ISO 10993-5 for the laboratories are insufficient to generate comparably sensitive tests.
Suggestion for the test setup
Based on the evaluation of the test parameters, we make the following suggestion for the test setup:
| Medium | MEM + 10 % Serum DMEM + 10 % Serum |
| Incubation | 24h pre-incubation, > 24h incubation 00h pre-incubation, 72h incubation |
| Assay | XTT BCA |
| Cells | L929 |
Conclusion
What does this mean for medical device manufacturers? A cytotoxicity test can be a very sensitive screening test. However, the test alone is not sufficient to evaluate biocompatibility!
To obtain high-quality test results, follow our power tips for laboratory commissioning.
7. Seven power tips for lab selection
Tip 1: ALWAYS specify all extraction parameters yourself.
Unfortunately, different laboratories or even the same employee in a laboratory can select different parameters for your device when re-testing it. For example, a change in the extraction ratio from 3 cm²/ml to 6 cm²/ml can result in the cytotoxicity being twice as high and vice versa. The same also applies for various other variables (see the figure above).
Tip 2: Where possible, always commission the same laboratory.
Since each laboratory has a slightly different test setup, you shouldn’t expect to get exactly the same results from two different laboratories. A second test in the event of poor results may, therefore, not be helpful.
So don’t be surprised by contradictory results. If your laboratory is accredited or GLP-certified, you should generally trust the laboratory’s results.
Tip 3: Always check the report and test results for deviations.
Nevertheless, even laboratories can make mistakes. Suboptimal device extraction can have a particularly big effect.
Outliers also regularly occur in the measurement results. If you have tested several devices in parallel, “mix-ups” are unfortunately not uncommon.
Tip 4: Always check the test results! This is the only way to discover trends.
You almost certainly check your devices for batch uniformity or at least at certain intervals for changes. So, you should urgently introduce monitoring to identify trends at an early stage.
For example, if you have a titanium product and cytotoxicity levels consistently around 15%, there may already be a major problem, even if the test is officially considered to be passed. This is because even low concentrations of residues can be above the relevant toxicological limits, even if these substances are almost not cytotoxic in the test.
Tip 5: Do not draw incorrect conclusions from failed tests.
Cytotoxic does not mean that you have not passed the test. There are no passes or fails in cytotoxicity testing.
With some materials, you will always get cytotoxic results. That doesn’t have to be a problem. It is well known, for example, that polyurethane is cytotoxic, and so are a lot of other materials.
A failed test does not mean that your device is not biocompatible. In such cases, a more detailed toxicological evaluation that goes beyond the material characterization required by ISO 10993-1 (Note: material certificates are not sufficient) is required.
Please email the Johner Institute’s experts, if you have any questions on this.
Tip 6: Do not draw incorrect conclusions from passed tests.
The cytotoxicity test alone is not sufficient to demonstrate purity or biocompatibility.
Do not assume that the cytotoxicity test will sufficiently detect every substance. For this reason, the test alone is not sufficient to prove the purity of medical devices or the absence of leachable substances.
Tip 7: Avoid unnecessary tests and testing costs.
Unsurprisingly, the cheapest test is not always the best. Of course, there are some very good laboratories with very low prices. However, paying low prices often only gets you a standard test that is not tailored to your device.
As always, you can tell if a laboratory is good or not by how quickly and actively they communication with you. Always ask about additional costs for:
- Non-sterile devices
- Sterilization
- Device handling
- GLP
- Test report
Sometimes cheap offers turn out to be a black box with considerable additional costs of up to 50%.
8. Conclusion
How you select your laboratory determines your test results at least as much as your device itself. And a passed cytotoxicity test does not mean that no substances of concern are released. By making sure your test parameters are tailored to your device and that they are correctly specified to the laboratory, you can achieve optimal comparability and ensure that you do not over or underestimate the cytotoxic effects of your device.
We will be happy to help you select a laboratory, determine the test parameters, commission the tests, and interpret the results if your device does not pass the test. Contact us by email or via our free micro-consulting service.
Change history
- 2025-12-17: Adjustments with regard to ISO 10993-1:2025.
- 2022-03-31: Chapter 6 added; results of the interlaboratory comparison.


