Process validation is the verification that a process meets the requirements imposed on its process results.
Learn when you must validate which processes (in the context of software) and how to ace validation. Furthermore, find out what process validation has to do with PQ, IQ, and OQ.
Process validation: What is it exactly?
a) Definition of the term “validation”
ISO 9000:2015 defines validation as follows
Confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been fulfilled
source: ISO 9000:2015
The standard remarks that the objective evidence necessary for validation is the result of a test or of another type of determination such as, for example, alternative calculations.
b) Definition of the term “process”
As for the definition of the term “process”, ISO 13485, again, refers to ISO 9000:2015.
set of interrelated or interacting activities that use inputs to deliver an intended result
source: ISO 9000:2015
Example processes are:
- development process
- sterilization process
- production process
- recruitment process
- sales process
We should further note that a process not only requires inputs and outputs (“results”), but also resources such as humans or machines (including software / IT).
c) Process validation
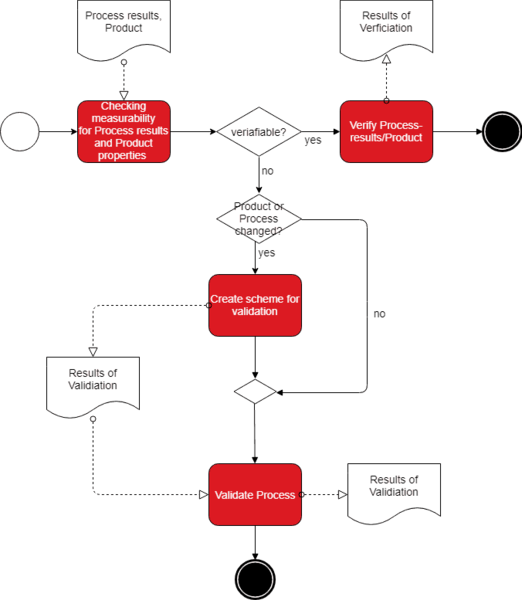
If we combine both definitions, it becomes clear that a process validation provides a confirmation of a process leading to the intended process results by objective evidence
For instance, in case of a development process one would ascertain that the development outcomes meet the requirements (“design input”). As for a sterilization process, one would ensure that the good to be sterilized actually is sterile.
The FDA explicitly defines the term:
Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications.
source: FDA
Tips for validating a process
a) Only validate relevant parameters
Usually, products have several characteristics manufacturers must ensure. The decision of whether a validation is necessary does not have to be made regarding a product / process, but regarding a characteristic (parameter). A very simplified example:
A syringe must be sterile and of a certain length. You can easily verify the parameter “length”. Therefore, you may not validate the process regarding whether the process results in syringes of the correct length, unless you don’t want to perform an exhaustive testing of the correct length.
On the contrary, you cannot ensure the parameter “sterility” by means of a “final inspection” since this would be destructive testing. Here, a process validation is necessary.
b) Validating a validation process?
If you perform an automated comprehensive testing of the length, this automated testing is also a process or process step which you must validate. If this process or process step comprises software or computer systems, you must validate those. An article on Computer System Validation CSV describes how you can proceed.
c) Verifying instead of validating
Even though a final testing is impossible in some cases, a testing of the product parameter, however, might already be possible in an intermediate step. If you, for example, can verify the correct assembly of a component, which is impossible to do after final assembly, the process might not necessarily be validated regarding whether the assembly of components was conducted correctly.
| Product Parameter | Process Step 1 | Process Step 2 | … | End Test | Validation necessary? |
| Parameter 1 | No testing required | No testing required | Testing performed | No | |
| Parameter 2 | No testing required | Testing performed | No testing possible | No | |
| Parameter 3 | No testing possible | No testing possible | No testing possible | No testing possible | Yes |
| … |
d) Process validation and PQ, IQ and OQ
Often, companies (especially in the pharmaceuticals sector) differentiate the following phases of process validation:
- IQ: This first tests at the site of the customer shall ensure that the device was delivered, installed, and built up according to specifications, that the device meets the users’ requirements, and that the documentation is present. At this stage, the basis for calibration, maintenance, and cleaning is provided. It is advised to have a look at the manual :-).
- OQ: During this most extensive testing, it should be checked whether the device operates according to specifications, especially at specification limits, to know what might happen in the worst case.
- PQ, then, is only the proof that the process runs under normal conditions. This test addresses, inter alia, measurement accuracy (incl. calibration and adjustment). It is also about long-term stability. In the pharmaceutical world, at least 3 batches are tested. A P-Diagram shows influencing factors such as environmental conditions, work steps, process parameters, and material properties. Here, sampling plans come into play.
Optimize your process validation and avoid problems during audits!
Few topics lead to as many discussions and problems during audits as process validation. With our e-learning videos and templates for compliant process validation, you can avoid these discussions and problems and shine in your next audit.
Regulatory requirements for process validation
a) Requirements of ISO 13485
ISO 13485:2016 requires process validation under the following circumstances, just as ISO 13485:2010 already did:
- The process is either a production or service process.
- The process outcomes can’t or aren’t verified, e.g., by means of measuring.
- The process outcome’s deficits would only be apparent when the product is used or after the service has been rendered.
If those conditions are met, process validation is required. For this purpose, the company must determine procedures including
- criteria to assess the process
- qualification of personnel
- application of methods and procedures
- documentation / records
- re-validation
- clearance of changes to a process
If software is used as part of these processes, this software must be validated.
b) Further relevant national and international provisions
The Global Harmonization Task Force GHTF, which no longer exists, has published the document GHTF SG 3 NB 99:10:2004 now “administered” by IMDRF.
Further, you should keep an eye on the Good Manufacturing Practices and the GAMP5 Guide.
c) Requirements of the FDA
The FDA lays out the requirements for process validation in the Quality System Regulations, more precisely in 21 CFR 820:75:
Process validation is only required if process outcomes cannot be verified. These validation activities must comprise:
- All activities which have been carried out must be recorded, including date and signature.
- Procedures, with which process parameters are surveilled, must be established.
- Only qualified personnel may validate a process.
- Methods and data used for controlling and monitoring processes, the date of execution, persons carrying out the validation, as well as relevant equipment must be documented.
- In case of changes, the manufacturer must assess whether re-validation is necessary and must carry it out if needed.
The FDA sets out the requirements for validating software in the “Guidance Document Software Validation”.
Contact us to find out what our experts can do for you.