ISO 20417:2021 Medical devices – Information to be supplied by the manufacturer establishes requirements for the general information that manufacturers have to supply with their medical devices and IVD devices. The authors have succeeded in presenting the criteria in a clear and comprehensible manner.
ISO 20417:2021 is also on the list of standards to be harmonized under the EU Medical Device Regulation (MDR). It is intended to replace EN 1041:2008+A1:2013, which described the requirements for the information to be provided under the EU Medical Device Directive (MDD). This means medical device manufacturers should be familiar with ISO 20417 and start applying it now.
This article will provide you with an overview of the standard and will look at the most important differences between it and EN 1041. This will enable you to quickly check the conformity of your accompanying information.
1. Scope of ISO 20417
a) Regulations taken into account by ISO 20417
In its introduction, the standard states that its aim is to serve as a central source of generally applicable information requirements. To do this it takes into account the requirements for the information to be provided that are established in the following regulations:
- IMDRF/GRRP WG/N47:2018 Essential Principles of Safety and Performance of Medical Devices and IVD Medical Devices
- IMDRF/GRRP WG/N52:2019 Principles of Labelling for Medical Devices and IVD Medical Devices on the information supplied by the manufacturer of a medical device
- MDR (EU) 2017/745
- IVDR (EU) 2017/746
b) Information provided by ISO 20417
ISO 20417 makes clear right at the beginning the types of information it wants to provide specifications for. It also provides some important term definitions.
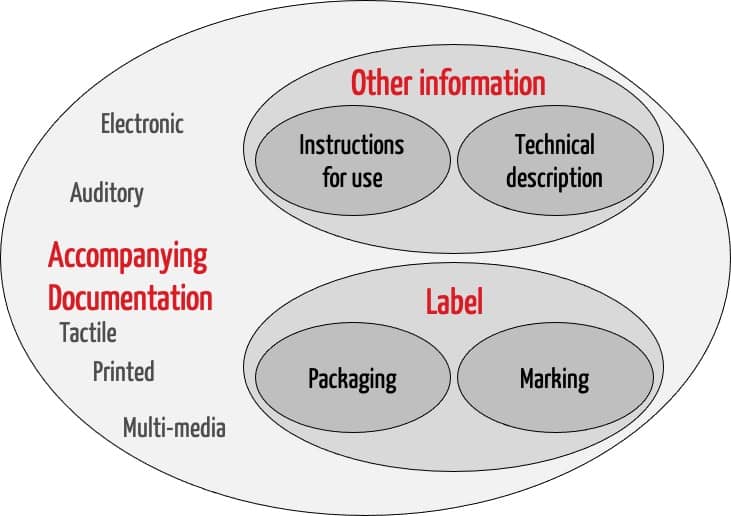
ISO 20417 is applicable for the accompanying information. This includes the label and other information. The label in turn consists of the information on the packaging and the marking on the device itself. The “other information” includes the instructions for use and the technical description.
2. Definitions of the terms “label” and “labeling” and the difference between them
a) Caution, term chaos!
The definition of the term label in ISO 20417 is essentially the same as in the MDR and IVDR. Unfortunately, the MDR and IVDR do not use the term label consistently, and sometimes use the term labeling synonymously. The leads to confusion as, in other relevant regulations, the term labeling means something different to label.
For example, ISO 13485 and the IMDRF use the term labeling as a synonym of accompanying information. So, in those cases, labeling refers to all the information supplied, including the label and IFU (Instructions For Use).
Read more about labeling medical devices.
In German, it is even more confusing because label and labeling are often both translated as “Kennzeichnung” (see ISO 13485).
The fact that ISO 20417 consistently uses the term accompanying information instead of labeling should prevent any confusion with the term label (unlike in the MDR/IVDR). The differentiation of different terms as well as the clear definitions in ISO 20417 should provide clarity and ensure a uniform understanding of the terms.
Whether the German translation of ISO 20417 can also do the same remains to be seen. The final version is not yet available in German. The German draft from 2019 doesn’t fill us with much hope.
b) Clarity through definitions
ISO 20417 helps to clear up the term chaos with numerous definitions.
“information accompanying or marked on a medical device or accessory for the user or those accountable for the installation, use, processing, maintenance, decommissioning and disposal of the medical device or accessory, particularly regarding safe use”
Source: ISO 20417
Although the standard does not use the term labeling, it does define the term label.
“means the written, printed or graphic information appearing either on the device itself, or on the packaging of each unit or on the packaging of multiple devices;”
Source: ISO 20417
The standard also provides definitions for the other terms listed in the Venn diagram in Fig. 1.
“information, in text or graphical format, durably affixed, printed, etched (or equivalent) to a medical device or accessory”
Source: ISO 20417
The instructions for use are a special kind of “other information”.
“package insert; portion of the accompanying information that is essential for the safe and effective use of a medical device or accessory directed to the user of the medical device”
Source: ISO 20417
This definition is not entirely consistent with the MDR definition:
“the information provided by the manufacturer to inform the user of a device’s intended purpose and proper use and of any precautions to be taken;”
Source: MDR (Article 2)
“Other information” also includes the technical description.
“portion of the accompanying information directed to the responsible organization and service personnel that is essential for preparation for the first use and safe use, maintenance or repair as well as processing, transport or storage for the expected lifetime of a medical device”
Source: ISO 20417
ISO 20417 does use the term labeling but ISO 13485:2016 does.
“label, instructions for use, and any other information that is related to identification, technical description, intended purpose and proper use of the medical device, but excluding shipping documents”
Source: ISO 13485:2016 3.8
Therefore, labeling according to ISO 13485 is most similar to the accompanying information defined by ISO 20417.
3. Chapter structure of ISO 20417
The chapter structure of ISO 20417 has been completely revised (compared to EN 1041) to accommodate the increased scope.
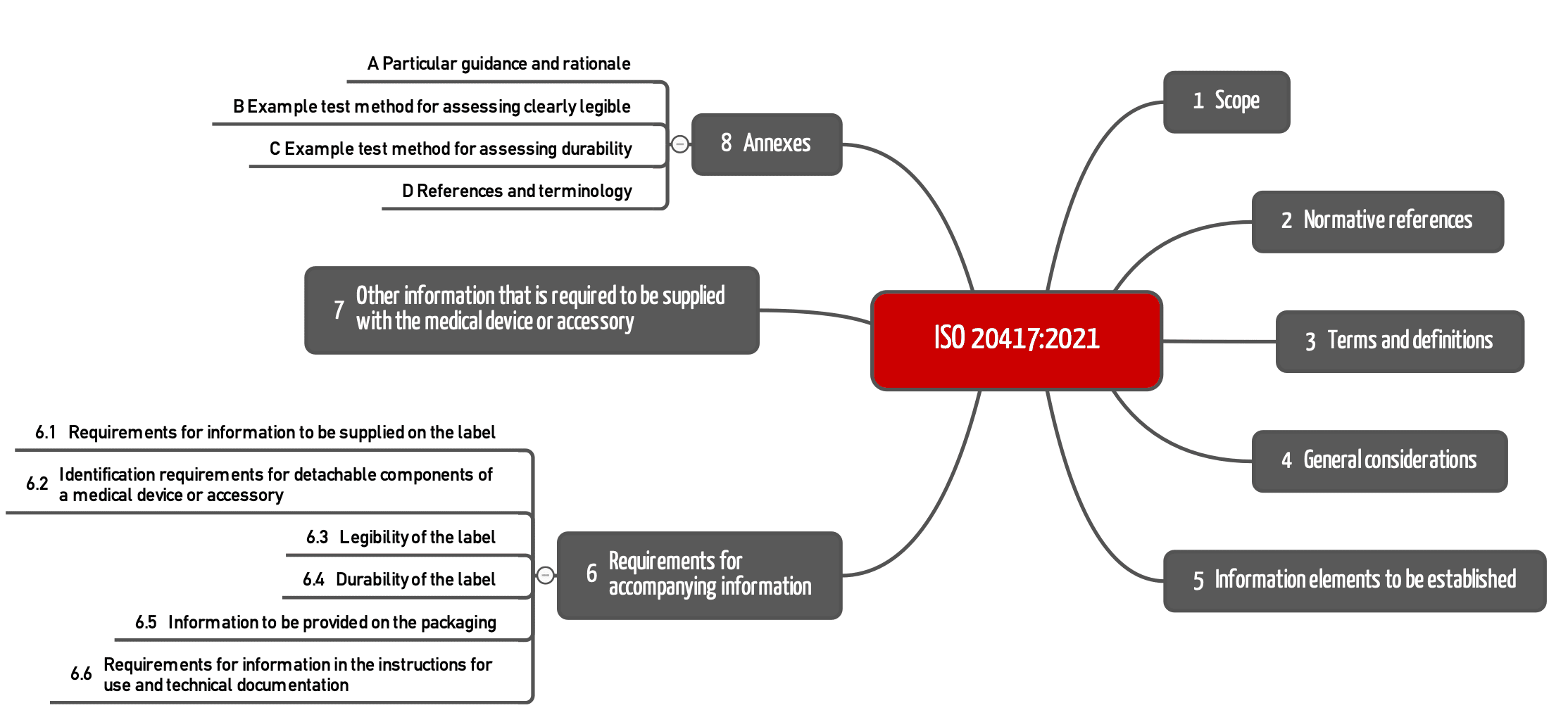
Chapter 4 and 5 establish general requirements for information in the accompanying documentation, i.e., for the label as well as the instructions for use and the technical description.
Chapter 6 lists specific requirements for the information:
- on the labels (6.1, 6.3, 6.4)
- on detachable device components (6.2)
- on the packaging (6.5)
- in the instructions for use (6.6)
Chapter 7 establishes further requirements for information about the:
- importer (7.1)
- distributor (7.2)
- repackaging (7.3)
- translation (7.4)
- regulatory identification (7.5)
The informative annexes contain useful guidance on legibility and durability, as well as references to the IVDR/MDR, the aforementioned IMDRF documents, and ISO 16142 parts 1 and 2.
4. Requirements of ISO 20417:2021 (selection)
Coming in at over 80 pages long, ISO 20417 has gained an enormous amount of content. This is due to the fact that all the requirements of the MDR/IVDR and the IMDRF documents have been integrated. Some requirements add to the MDR/IVDR requirements, others provide clarification and provide helpful guidance. Some examples are highlighted below.
a) Risk management and usability engineering
The risk management and usability engineering processes should identify the information the accompanying information needs to contain in order to encourage safe use of the device.
The information must be designed to be comprehensible for the intended users (see Chapter 4).
b) Use of symbols
It is not clear from the MDR whether symbols have to be used for the label. In Chapter 5.2, “Graphical information”, ISO 20417 makes clear that the information on the label can be given either as text or using symbols. If symbols are used, the standard specifies the standards these symbols should be taken from (e.g., ISO 15223-1). Unless otherwise required by law, these symbols should be explained in the instructions for use.
The Johner Institute recommends always describing the symbols used in the instructions for use, as the meaning of some symbols may not be clear to lay users.
c) Language codes
The language codes from ISO 639 parts 1-3 can be used to label the languages used in multilingual information (see Chapter 5.3).
d) Date
The date must be given in the format YYYY-MM-DD or YYYY-MM or YYYY (see Chapter 5.4).
e) Label on the device itself or on the packaging
The MDR states, somewhat vaguely, that the label must be on the device itself or, “if this is not practicable or appropriate”, it may be provided on the packaging (see MDR, Annex I 23.1 (b)). In Chapter 6.1.1, ISO 20417 specifies that the label doesn’t have to be on the device itself only if:
- the dimension of the device does not allow it
- the surface material of the device does not allow it or
- not providing the information on the device itself does not lead to risks
f) eIFU
If electronic instructions for use are provided instead of instructions for use on paper, this must be stated on the label. The appropriate symbol from ISO 15223-1 can be used to do this (see Chapter 6.1.3 (d)).
g) Nanotechnology
If the device contains nanomaterials, this should be stated on the label. The appropriate symbol from ISO 15223-1 can be used to do this (see Chapter 6.1.3 (e)).
h) Instructions for use as a risk control measure
If the instructions for use are part of the risk control measures, the label must state that the instructions for use must be read (see Chapter 6.1.5).
i) Identification of detachable device parts
According to ISO 20417, Chapter 6.2, if a lack of identification of detachable device parts would generate a risk, these parts must be given their own abbreviated label with the following information:
- manufacturer name (without address)
- device ID
- batch number
j) Information on the packaging
In addition to the marking on the device itself, information such as the manufacturer (incl. address), device identification, etc. must also be provided on the packaging (see Chapter 6.5).
k) Instructions for use minimalism
The instructions for use should contain only the information required according to the regulations and all additional information that the user needs to use the device safely and effectively. Information that is not required by the user or is not required by the regulations should be omitted.
This information can be given in the technical description instead (see Chapter 6.6.2 b).
l) Providing information on the website
The MDR states a bit vaguely:
“Each device shall be accompanied by the information needed to identify the device and its manufacturer, and by any safety and performance information relevant to the user, or any other person, as appropriate. Such information may appear on the device itself, on the packaging or in the instructions for use, and shall, if the manufacturer has a website, be made available and kept up to date on the website, taking into account the following: […]”
MDR, Annex I, (23.1)
Does this mean that the entire instructions for use must be available on the website or only the parts concerning safety and performance?
ISO 20417 states that the entire instructions for use should be made available on the website (but they don’t have to be; see Chapter 6.6.5 (a)). So, it doesn’t provide much clarity either.
m) Information in the event of repackaging and translation
If the device has been repackaged by someone other than the manufacturer or the label or instructions for use have been translated (for example, if it is imported), the name and address of this third party must be given on the label. The manufacturers can use the corresponding symbols from ISO 15223-1 to do this.
5. Differences from EN 1041
Chapter 4, “Requirements”, of EN 1041 is divided into two chapters (which now contain significantly more requirements) in ISO 20417:
- 4 “General considerations”
- 5 “Information elements to be established”
Chapter 5 of EN 1041, “Requirements for provision of information”, has been integrated into Chapter 6 of ISO 20417, “Requirements for accompanying information.” It now has the following sub-chapters:
- 6.1 “Requirements for information to be supplied on the label”
- 6.2 “Identification requirements for detachable components of a medical device or accessory”
- 6.5 “Information to be provided on the packaging”
- 6.6 “Requirements for information in the instructions for use and technical description”
The Chapters
- 6.3 “Legibility of the label” (“Legibility” in EN 1041) and
- 6.4 “Durability of markings” (“Availability” in EN 1041)
in ISO 20417 will already be familiar from EN 1041 but have increased in scope.
Chapter 5.2.2 “Accessibility” in EN 1041 is now in Chapter 4(e) of ISO 20417, Chapter 5.2.5 “Security” has been omitted. Unlike EN 1041, ISO 20417 does not explicitly require the label to be protected against corruption and intentional modification.
Chapter 5.2.6 of EN 1041, “Changes to information provided”, is also not included in ISO 20417. Changes to the information provided now do not necessarily have to be reported to existing users according to ISO 20417. Chapter 6 “Documentation” has also been omitted.
6. Conclusion
ISO 20417 summarizes the requirements of various relevant regulations in a clear and comprehensible manner. It clarifies some requirements of the MDR/IVDR that are not very clear and gives practical guidance on how to comply with them, making it easier for manufacturers to meet the requirements of the MDR/IVDR and other regulations efficiently.
Terms are clearly defined and differentiated, and this should help to prevent confusion, e.g., between the terms label and labeling, and ensure a common understanding of the terms.
ISO 20417 offers manufacturers real guidance on how to meet the requirements for the accompanying information.
Manufacturers would be well advised to review their accompanying information using ISO 20417 and, if they have them, update their accompanying information (labeling) checklists.
Do you need help reviewing your label or instructions for use, or creating your instructions for use? Then please contact us.

